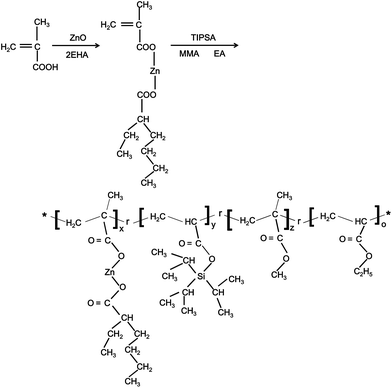
Synthesis of hybrid zinc/silyl acrylate copolymers and their surface properties in the microfouling stage†
Rongrong Chenab,
Yakun Lia,
Minglong Yana,
Xun Suna,
Huajing Hana,
Jie Lia,
Jun Wangab,
Lianhe Liu*abc and
Kazunobu Takahashi*ab
aKey Laboratory of Superlight Material and Surface Technology, Ministry of Education, Harbin Engineering University, 150001, China. E-mail: kazunobu@163.com; liulianhe@hrbeu.edu.cn
bInstitute of Advanced Marine Materials, College of Materials Science and Chemical Engineering, Harbin Engineering University, Harbin 150001, China
cQingdao Advanced Marine Material Technology Co., Ltd, Qingdao, 266100, China
First published on 27th January 2016
Abstract
Development of an environmentally friendly and efficient marine antifouling coating is a central goal in marine antifouling. In this study, a series of novel hybrid zinc/silyl acrylate copolymers (Zn/Si-acrylate copolymers) composed of tri(isopropyl)silyl acrylate (TIPSA), zinc-2-ethylhexanoate methacrylate (Zn-monomer), ethyl acrylate and methyl methacrylate were synthesized and their surface compositions, thermal degradation and hydrolysis properties were investigated. After being immersed in seawater, the hydrolysis of TIPSA and Zn-monomer could lead to a gradual self-peeling of the Zn/Si-acrylate surfaces, which was controlled by the ratio of TIPSA to Zn-monomer. The Zn/Si-acrylate copolymers with high Zn-monomer content showed excellent performance in the resistance of diatom Phaeodactylum tricornutum (P. tricornutum) growth on the Zn/Si-acrylate films. Both the self-peeling and release of zinc compounds lead to antifouling properties, which demonstrated that Zn/Si-acrylate copolymers were an effective resin for marine antifouling in static or low flow conditions.
1. Introduction
Marine biofouling, which has been an economic problem for centuries, leads to an increase in the shear drag on ships' hulls with environmental and economic penalties, accelerates the corrosion of metallic substrates and decreases the service life of marine equipment.1,2 Chemically active marine coatings containing tributyltin (TBT) and cuprous oxide are effective for combating marine fouling, but they are under environmental scrutiny because they are detrimental to nontarget organisms and ecologically harmful.3–5 As a consequence, research efforts have focused on environmentally friendly systems, such as self-polishing polymers,6,7 amphiphilic polymers,8–11 zwitterionic polymers,12,13 biodegradable copolymers,14,15 and hydrogels,16,17 that are able to repel marine fouling organisms or minimize their adhesion to surfaces.Among these applicable antifouling paints, self-polishing coatings with acrylate esters are one type of the most useful, effective and popular antifouling coatings. Since the worldwide prohibition of TBT for its high adverse effects on marine environments,18 a series of zinc acrylate copolymers and triorganosilyl methacrylate-based polymers bearing hydrolysable pendant groups have been used as substitutes.19,20 All acrylate copolymers are initially water-insoluble and able to be hydrolyzed in seawater through an ionic exchange or hydrolysis reaction with seawater, which make them water-soluble. The hydrolysis of alkylsilyl (meth)acrylate-based polymers could give the coatings self-smoothing properties, which positively contribute to the hydrodynamics of the ship's hull by reducing the frictional drag and total amount of fuel consumed.21 Bressy et al. first reported hydrophobic–hydrolysable copolymers consisting of methyl methacrylate (MMA) and tert-butyldimethylsilyl methacrylate (TBDMSMA) synthesized by a reversible addition-fragmentation chain transfer (RAFT) polymerization technique, and the diblock copolymers exhibited good antifouling abilities in the Mediterranean Sea for 18 months.6,22,23 Hong et al. reported a novel type of self-hydrogel-generating antifouling coating made of cross-linked copolymer chains containing methyl methacrylate (MMA), acrylic acid (AA) and hydrolysable tributylsilyl methacrylate (TBSMA).24
These reports indicated that the leaching form of the polymers depended on the hydrolysable monomer content,22,25 size of the alkyl groups linked to the silicon atom,26 molecular weight and hydrophilicity of the comonomers.23,27 However, the alkyl-silyl methacrylate-based paint with a low erosion rate exhibited poor antifouling ability in a static marine environment unless the alkylsilyl (meth)acrylate content was increased to over 45 wt%.3,28 Furthermore, the coatings with this copolymer as the binder also perform poorly against diatoms, the major component of primary colonizers or slime, which is the key step for the colonization of spores and larvae of macrofoulants including macroalgae, sponges, barnacles, bryozoans, tunicates and so on.29,30 This situation requires the removal of fouling with a higher speed from extensively fouled hulls. Therefore, it is important to optimize the structure of a alkylsilyl (meth)acrylate copolymer to improve its antibiofouling ability under static or low speed conditions.
In addition, little is known about Phaeodactylum tricornutum (P. tricornutum) cell adhesion properties and the surface elements related to the hydrolysis of polymers for self-polishing antifouling coatings. In this study, a set of antifouling binders were developed to form a self-renewing and anti-diatom surface using Zn/Si-acrylate copolymers composed of different ratios of tri(isopropyl)silyl acrylate (TIPSA), zinc-2-ethylhexanoate methacrylate (Zn-monomer), ethyl acrylate (EA), and methyl methacrylate (MMA) (as shown in Scheme 1). By varying the monomer ratio of TIPSA to Zn-monomer, copolymers with a wide range of compositions could be acquired. The function of the labile ester, including organosilyl ester and zinc carboxylic ester, on the thermal degradation and hydrolysis properties were investigated and compared to the TIPSA copolymer for the first time, under the same conditions. The inhibition of the growth of diatoms and adhesion experiments showed that the Zn/Si-acrylate copolymer manifested a strong resistance to fouling.
2. Experimental
2.1 Materials
Xylene, 1-methoxy-2-propanol (PGM), EA, MMA, 2-ethylhexanoic acid (2EHA), methacrylic acid (MAA), and azobisisobutyronitrile (AIBN) were purchased from the Guangfu Chemical Reagent Co. (Tianjin, China). Tri(isopropyl)-silyl acrylate (TIPSA) and benzoyl peroxide (BPO) were obtained from the Shanghai Novel Technical Co. and the Shanghai Shanpu Chemical Co., respectively. Deionized water was purified with a Milli-Q Plus system (Millipore, Schwalbach, Germany). Artificial seawater (ASW) used in diatom cultivation was prepared according to ASTM D1141-98, whereas the ASW in the film immersion experiment was prepared by reef salt provided by Seachem. All reagents were used as received without further purification.2.2 Synthesis of the copolymer resin
Zn/Si-acrylate copolymers were synthesized by a two-step method as shown in Scheme 1. First, ZnO, MAA and 2EHA were used to synthesis Zn-monomer by an acid–base reaction; then, Zn/Si-acrylate copolymers were prepared via radical copolymerization initiated by AIBN and BPO. AIBN was dissolved with the monomers and initiated polymerization in the first stage and BPO was added in the second stage to increase the monomer conversion degree as chaser.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1600 (O
O), 1600 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O).
C–O).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 883 (Si–C) cm−1.
O), 883 (Si–C) cm−1.
| Sample | MMA/EA/TIPSA/Zn-monomera | Mnb/g mol−1 | PDI | Conversion/% |
|---|---|---|---|---|
| a Feed weight ratio.b Determined by GPC with PS standards. | ||||
| Si-polymer | 10/52/38/0 | — | — | 96 |
| Zn/Si-1 | 10/52/34/4 | 1200 | 1.09 | 95 |
| Zn/Si-2 | 10/52/31/7 | 3200 | 2.43 | 92 |
| Zn/Si-3 | 10/52/28/10 | 5000 | 1.76 | 83 |
| Zn/Si-4 | 10/52/25/13 | 3700 | 2.22 | 95 |
| Zn/Si-5 | 10/52/22/16 | — | — | — |
| Zn/Si-6 | 10/52/19/19 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
2.34 | — |
2.3 Characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1).
000 g mol−1).2.4 Biofouling assays
The diatom, P. tricornutum ACCC 01625 was obtained from the Center for Collections of Marine Algae of Xiamen University (CCMA), Pearl River Estuary, China. Cells were cultured in F/2 medium without aeration at 21 ± 2 °C with a 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 h light
12 h light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark (L/D) cycle of fluorescent illumination at 2000 lux,31 and they were gently stirred twice daily. A log-phase suspension of cells was found to have a cell number of approximately 105 cells per mL. One hundred mL of cell suspension was added to individual conical flasks, each containing a test slide, prepared as described above. The microalgae cell concentration was counted on a cell count chamber hemocytometer with an optical microscope (Leica DML 300B, Germany) and correlated to the optical densities at 314.5 nm of the microalgae samples based on linear fitting equations. For every period, the micro-algal density was quantified via a UV-2550-Visible Spectrophotometer (SHIMADZU Corporation, Japan). All of the experiments were carried out in three replicates. The error bars correspond to the average standard deviations. The cells attached to the surfaces were observed using an optical microscope (Leica dmi 300B).
dark (L/D) cycle of fluorescent illumination at 2000 lux,31 and they were gently stirred twice daily. A log-phase suspension of cells was found to have a cell number of approximately 105 cells per mL. One hundred mL of cell suspension was added to individual conical flasks, each containing a test slide, prepared as described above. The microalgae cell concentration was counted on a cell count chamber hemocytometer with an optical microscope (Leica DML 300B, Germany) and correlated to the optical densities at 314.5 nm of the microalgae samples based on linear fitting equations. For every period, the micro-algal density was quantified via a UV-2550-Visible Spectrophotometer (SHIMADZU Corporation, Japan). All of the experiments were carried out in three replicates. The error bars correspond to the average standard deviations. The cells attached to the surfaces were observed using an optical microscope (Leica dmi 300B).
2.5 Weight-loss measurement
To measure the self-peeling rates, each dried coated glass slide was weighed (w0) before dipping into a tank of artificial seawater (ASW) at 21.6 ± 1 °C that was changed every week. After different time periods, the glass slides were taken out from the ASW, immersed in distilled water for 4 h and then gently dried at room temperature before their weights (wt,wet) were recorded. Then, all of these glass slides were dried at 100 °C for 6 h before their weights (wt,dry) were recorded. The weight difference between wt,wet and w0 reflects the water adsorption of each coating, whereas the weight difference between wt,wet and wt,dry shows the weight-loss (self-polishing or self-peeling) of water in each coating's leaching layer. For each resin, three coated glass slides were prepared and tested. Therefore, each result reported and used hereafter was averaged over three identical coatings. The mass loss (%) was calculated from eqn (1):
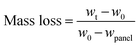 | (1) |
3. Results and discussion
3.1 The chemical compositions of the Zn/Si-acrylate copolymers
In the present work, Zn/Si-acrylate copolymers were synthesized by a two-step method as shown in Scheme 1. The Zn-monomer was first synthesized and copolymerized with TIPSA and other monomers. The chemical structures of Zn-monomer, TIPSA, Si-polymer and Zn/Si-acrylate copolymers by FT-IR spectra are shown in Fig. 1. The peaks at 1630–1620 cm−1 and 880 cm−1 (Fig. 1a) were ascribed to the stretching vibrations of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and Si–C bonds, respectively. The peak intensity of the C
C and Si–C bonds, respectively. The peak intensity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in copolymers decreased significantly compared to that in monomers (Fig. 1b), whereas the peak at 1730 cm−1 corresponding to the carbonyl group of ester bonds in the Zn/Si-acrylate copolymer chains was observed. Comparing the spectrum of Si-polymer with that of Zn/Si-acrylate copolymers, we can find that the asymmetrical stretching vibration of the carboxylic group showed a peak at approximately 1600 cm−1. The relative intensities of 1600 cm−1 and 800 cm−1 increased with the Zn-monomer and TIPSA content, respectively. These results suggested that the TIPSA monomer and Zn-monomer were copolymerized.
C bonds in copolymers decreased significantly compared to that in monomers (Fig. 1b), whereas the peak at 1730 cm−1 corresponding to the carbonyl group of ester bonds in the Zn/Si-acrylate copolymer chains was observed. Comparing the spectrum of Si-polymer with that of Zn/Si-acrylate copolymers, we can find that the asymmetrical stretching vibration of the carboxylic group showed a peak at approximately 1600 cm−1. The relative intensities of 1600 cm−1 and 800 cm−1 increased with the Zn-monomer and TIPSA content, respectively. These results suggested that the TIPSA monomer and Zn-monomer were copolymerized.
 | ||
| Fig. 1 FTIR spectra of the Zn-monomer and TIPSA (a). FTIR spectra of Si-polymer and Zn/Si-acrylate copolymers (b). (1) 1600 cm−1, (2) 880 cm−1. | ||
Fig. 2 illustrates the TG and DTGA curves of Si-polymer and hybrid Zn/Si-acrylate copolymers at a heating rate of 20 K min−1 under an inert atmosphere. The Si-polymer exhibits only a main decomposition at high temperature from 350 to 440 °C. However, for Zn/Si-acrylate copolymer samples, three weight loss stages were encountered in the thermal degradation, with a main decomposition, which occurred from 350 to 450 °C. A first weak degradation stage and the third stage of weight loss were recorded from 180 to 310 °C and from 455 to 490 °C, respectively. Compared with Si-acrylate copolymers and previous related reports, the first slight DTG peak from approximately 207 to 270 °C would correspond to the degradation initiated by a radical transfer to the unsaturated chain. The second well-resolved sharp peaks between 395 and 425 °C could be indicative of a degradation initiated from the random C–C scission of the backbone for Zn/Si- and Si-acrylate copolymers.15,23,32 The third stage of Zn/Si-acrylate co-polymers thermal degradation at 475 °C would correspond to the random chain scission of methacrylic acid zinc salt saturated polymer chains because the depropagation rate is in keeping with the content of the carboxylic acid metal salt component. Compared with Si-polymer, these hybrid Zn/Si-acrylate copolymers produced significant amounts of residual char in nitrogen in 490 °C due to the existence of Zn-monomer. Therefore, all the information indicated that the TIPSA monomer was successfully copolymerized with Zn-monomer in the reaction.
3.2 Hydrolysis of Zn/Si-acrylate copolymers
Fig. 3 shows the immersion-time dependence of the mass loss of Zn/Si-acrylate copolymers in artificial seawater under static conditions. Both the wet mass loss (Fig. 3a) and dry mass loss (Fig. 3b) display similar trends. The films with different molar proportions of hydrolysable units within the macromolecular chains behave similarly, indicating that at the initial stage (approximately 445 h), all of the films tested start to lose weight. After approximately 400 h of immersion, the weight loss rates of all of the films become stable. Both the relative weight loss of the hybrid coatings and the self-peeling rate (the slope) all increase with the Zn-monomer content. According to the report,20 the amount of zinc acrylate and the type of the alkyl groups in the acrylic ester comonomers had the most significant influence on the leaching of the polymers together. This may be because the Zn-monomer in the acrylate copolymers has a higher hydrolysis rate than TIPSA, which makes those copolymer chains on the top layer soluble so that they are washed away at a faster rate. In comparison with cross-linked terpolymer with hydrolyzable tributylsilyl methacrylate (TBSM) or triisopropylsilyl methacrylate (TIPSM),7 the Zn/Si-acrylate films lost more weight than cross-linked terpolymer after immersion, which is due to that fact that TIPSA has a higher hydrolysis rate than TBSM or TIPSM. The higher self-peeling process could lead to an excellent antifouling property in static water environment.Fig. 3c showed the mass loss differences of the wet weight and dry weight of Si- and Zn/Si-acrylate copolymers for different immersion times. Within the initial 250 h, the mass loss difference of wet weight and dry weight decreased sharply, and the situation tended to steady after 250 h. For a random copolymer, the zinc and silyl monomer units were statistically distributed all along the macromolecular chains with a slight deviation in the molar composition during the polymerization, as previously reported.33 The formation of carboxylate ions could occur uniformly and result in the hydrolyzed polymer. The mass loss difference indicated the quantity of water absorption of the hydrophilic surface layers which were made by the hydrolysis of Zn-monomer and TIPSA. The initial water absorption led to the swelling of the top layer of each film to form a hydrogel and not the dissolution in ASW for the long chain. The average mass loss difference after 250 h of immersion in ASW increased with the Zn-monomer content from 0.005% to 0.02% (Fig. 3d). Concretely, the water content trapped in films increased linearly with the increase in the Zn-monomer molar content to 5% and the decrease in the silyl acrylate monomer from 16.6 to 9.6%. Meanwhile, when the Zn-monomer content is over 5.5%, the absorption began to decrease. The results indicated that the hydrolysis of Zn-monomer could accommodate more amount water than TIPSA monomer in the leached layers, and also provided higher weight loss of the polymers combined with the result of Fig. 3a and b. This suggested that the hydrolysis of TIPSA and Zn-monomer eventually made a thin hydrophilic surface layer, which is gradually dissolved and washed away, further exposing the inner layer to sea water when the polymer surface is immersed in seawater. Therefore, the balance between the hydrolysis and dissolution of the Zn/Si-acrylate copolymer is critical.
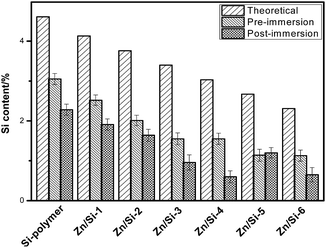
No studies have been devoted to the hydrolysis/ionic exchange reaction of hybrid Zn/Si-acrylate copolymers. The synthesized Zn/Si-acrylate copolymers were fabricated into flat films via the membrane scraper. Fig. 4 shows the EDS analysis of the surfaces of the Si-polymer and the Zn/Si-acrylate co-polymers pre- and post-immersion in the diatom solution. A comparison of the experimental EDS composition data of the copolymers with the theoretical values demonstrates that the surface is not dominated by the tri(isopropyl)silyl moieties for the short side chains and homopolymerization of all of the monomers. The chemical compositions of the Zn/Si-acrylate copolymer surfaces immersed in the diatom solution showed lower elemental Si contents.
To obtain more information about these hydrolysable surfaces, XPS analysis was used to determine the chemical composition and nature of the chemical bonding on the surfaces. Fig. 5 shows the X-ray photoelectron spectra (XPS) of pristine Si-acrylate and Zn/Si-acrylate films as well as their higher resolution spectra of the C 1s areas. The binding energies in the XPS experiment were corrected by referencing the C 1s peak to 284.6 eV. In the XPS wide-scan spectra of the pristine Si-polymer (Fig. 5a) and Zn/Si-3 film (Fig. 5c), the Si 2p and Si 2s signals with respective biding energies (BEs) of approximately 99 and 151 eV are associated with alkoxysilanes and their elemental composition. The depicted elemental Zn with binding energies at approximately 1022 and 1045 eV shown as an inset in Fig. 5c indicates the successful copolymerization of Zn-monomer and TIPSA.34 These results agreed well with the EDS data and demonstrated a decrease in the silicon and Zn atoms within the surface prior to the immersion. The C 1s core-level spectrum of pristine Si-polymer (Fig. 5b) can be fitted into four components: the main peak at 284.6 eV was due to carbon bound only to carbon and hydrogen (C–H/C–C); the other three fitted peaks were attributed to the component O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (i.e., carboxylic esters) species at 288.4 eV, C–O species at 286.2 eV and Si–C species at 283.9 eV.35,36 The area ratios of the four peak components [C
O (i.e., carboxylic esters) species at 288.4 eV, C–O species at 286.2 eV and Si–C species at 283.9 eV.35,36 The area ratios of the four peak components [C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O]
O]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [C–O]
[C–O]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [C–H]
[C–H]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [C–Si] are approximately 1
[C–Si] are approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.8
4.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and 1
0.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.8
5.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3, which are consistent with the theoretical ratios of the Si-polymer and Zn/Si-acrylate copolymers.
0.3, which are consistent with the theoretical ratios of the Si-polymer and Zn/Si-acrylate copolymers.
In comparison to the wide scan spectrum of the pristine surface (Fig. 5a and c), the weak Cl 2p (at the BE of approximately 209 eV), Na 1s (at the BE of approximately 1078 eV) and N 1s (at the BE of approximately 400 eV) signals appear in the wide scan spectra of the Zn/Si-acrylate surfaces (Fig. 6a and c), which may be caused by the absorption of the ions in artificial seawater. The C 1s core-level spectra of pristine Si-polymer and Zn/Si-3 (Fig. 6b and d) can be curve-fitted into three peak components with BEs at approximately 288.4, 286.2 and 284.6 eV, attributable to the O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (i.e., carboxylic acid and esters), C–O and C–H species, respectively. The area ratios of the three peak components [C
O (i.e., carboxylic acid and esters), C–O and C–H species, respectively. The area ratios of the three peak components [C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O]
O]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [C–O]
[C–O]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [C–H] are approximately 1
[C–H] are approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8
1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.6 and 1
3.6 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4
1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.7, which indicated a decrease in the carbonyl groups. Additionally, in the post-immersed surfaces, Si–C species were not detected at 283.9 eV.
4.7, which indicated a decrease in the carbonyl groups. Additionally, in the post-immersed surfaces, Si–C species were not detected at 283.9 eV.
 | ||
| Fig. 6 Survey curves of (a) Si-polymer immersed in ASW, its higher resolution curves of C 1s (b), (c) Zn/Si-3 immersed in ASW and its higher resolution curves of C 1s (d). | ||
Thus, the degradation (weight loss) speed of the coating is mainly controlled by both the contents of TIPSA and Zn-monomer, which can significantly improve the dissolution after hydrolysis, leading to enhanced antibiofouling in static marine environments. It is also important to note that in our design, the degradation products released into seawater are mainly fragmented poly(methyl methacrylate) with some anionic carboxylate groups, triisopropylsilyl cations and PMMA–EA copolymer chains. To the best of our knowledge, they are not harmful and are nontoxic in marine environments.
3.3 Diatom growth inhibition and adhesion assays
Diatoms are a major component of microbial slimes that develop on man-made surfaces placed in marine environments.37 Generally, the biofouling starts from the adhesion of bacteria, diatoms and other microalgae on the artificial surfaces immersed in the marine environment. Therefore, the diatom P. tricornutum was used to evaluate the antifouling character of the Zn/Si-acrylate copolymer surfaces. The effect of Zn/Si-acrylate copolymers on the inhibition of growth was evaluated in the P. tricornutum suspension. Fig. 7 shows the number of diatom cells in the suspension with different Zn/Si-acrylate copolymers after cultivation for 5, 19, 24, 48, 72, 120 and 168 h. All of the polymer surfaces have a growth inhibitory behavior as the blank sample showed the highest cell density. It was observed that the growth rate of the diatom declined with an increase in the Zn-monomer content, which may be due to the fragment with some anionic carboxylate groups and the zinc-2-ethylhexanoic cation.The XPS wide scan spectra of Si-polymer, Zn/Si-3 and Zn/Si-6 surfaces after 15 d of exposure to the P. tricornutum cell solution and 30 min in a deionized water bath are shown in Fig. 8. The weak N 1s signals appear in the wide scan spectra of the Zn/Si-acrylate surfaces (Fig. 8a, c and e), which are suspected to be caused by the absorption of elemental N from substitute ocean water, F/2 culture medium or the exocellular polysaccharides, which may act as metal chelating ligands and were produced to alleviate metal stress.38 The [N]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [C] ratio, determined from the N 1s and C 1s core-level peak area ratio, is used to indicate the relative amounts of nitrate and proteins adsorbed on the surface.39 From the comparison of the XPS wide-scan spectra, the relative intensity of the N 1s signal of the Zn/Si-acrylate surface is much stronger than that of the Si-polymer surface. Quantitative peak analysis showed that the [N]
[C] ratio, determined from the N 1s and C 1s core-level peak area ratio, is used to indicate the relative amounts of nitrate and proteins adsorbed on the surface.39 From the comparison of the XPS wide-scan spectra, the relative intensity of the N 1s signal of the Zn/Si-acrylate surface is much stronger than that of the Si-polymer surface. Quantitative peak analysis showed that the [N]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [C] molar ratio at the Zn/Si-6 surface is 0.056, in comparison with that at the Si-polymer surface, which is 0.001. This increase is probably due to Zn complexation with native EPS cells in the extracellular polymeric substance (EPS) Zn/Si-acrylate copolymer surface and the rough surfaces of Zn/Si-acrylate films.34 Although the Si-polymer surface has little effect on the inhibition of the growth of diatoms, it also has a weak adhesion effect for the exocellular polysaccharides.
[C] molar ratio at the Zn/Si-6 surface is 0.056, in comparison with that at the Si-polymer surface, which is 0.001. This increase is probably due to Zn complexation with native EPS cells in the extracellular polymeric substance (EPS) Zn/Si-acrylate copolymer surface and the rough surfaces of Zn/Si-acrylate films.34 Although the Si-polymer surface has little effect on the inhibition of the growth of diatoms, it also has a weak adhesion effect for the exocellular polysaccharides.
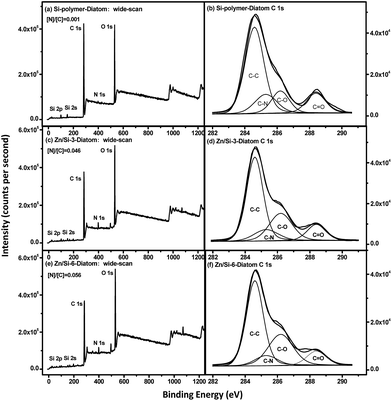 | ||
| Fig. 8 XPS wide scan and C 1s core-level spectra of (a and b) Si-polymer, (c and d) Zn/Si-3 and (e and f) Zn/Si-6 immersed in the diatom solution. | ||
The XPS C 1s core-level spectra of the Si-polymer, Zn/Si-3 and Zn/Si-6 surfaces can be curve-fitted into four peak components with BEs of approximately 284.6, 285.6, 286.2 and 288.4 eV (Fig. 8b, d and f), which are attributable to the C–H, C–N, C–O, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species, respectively.40,41 From the quantitative changes in the peak area ratios, it can be seen that the contents of O
O species, respectively.40,41 From the quantitative changes in the peak area ratios, it can be seen that the contents of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O groups on surfaces that were immersed in artificial seawater and the diatom solution are less than the as-prepared surface. This result demonstrates that O
C–O groups on surfaces that were immersed in artificial seawater and the diatom solution are less than the as-prepared surface. This result demonstrates that O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O groups in the methacrylic acid zinc salt component and carboxylic silyl ester component decreased in the artificial seawater. According to Fig. 5, 6 and 8, we can discover that in both the artificial seawater and diatom solution, the O
C–O groups in the methacrylic acid zinc salt component and carboxylic silyl ester component decreased in the artificial seawater. According to Fig. 5, 6 and 8, we can discover that in both the artificial seawater and diatom solution, the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O groups and C–O bonds appeared on the reacted surfaces. This finding is in good agreement with the hydrolysis, i.e., the surface of which is mainly occupied by carbonyl moieties. Due to a hydrolysis/ionic exchange reaction with seawater, the zinc- and trialkylsilyl-pendant groups will be cut off from the backbone. Thus, based on the XPS analysis, we could deduce that both the methacrylic acid zinc salt component and carboxylic acid silyl ester component are changed in the seawater medium, especially in a diatom solution. A conclusion can be drawn that two factors, the hydrolysable monomer type and the content of this monomer in the copolymers, are crucial in the preparation of copolymer films with controlled hydrolyzed antifouling polymer.
C–O groups and C–O bonds appeared on the reacted surfaces. This finding is in good agreement with the hydrolysis, i.e., the surface of which is mainly occupied by carbonyl moieties. Due to a hydrolysis/ionic exchange reaction with seawater, the zinc- and trialkylsilyl-pendant groups will be cut off from the backbone. Thus, based on the XPS analysis, we could deduce that both the methacrylic acid zinc salt component and carboxylic acid silyl ester component are changed in the seawater medium, especially in a diatom solution. A conclusion can be drawn that two factors, the hydrolysable monomer type and the content of this monomer in the copolymers, are crucial in the preparation of copolymer films with controlled hydrolyzed antifouling polymer.
P. tricornutum cells, unlike the spores of Ulva, are not motile in the water column. The cells come into contact with a surface by gravity and water currents. Therefore, approximately the same number of diatoms will be in contact with all test surfaces during the incubation period. Fig. 9 shows the optical microscopy images of thin films of Zn/Si-acrylate copolymer (Fig. 9a–f) immersed in the suspension after 10 d and images of the test diatom with predominantly a fusiform morphology (Fig. 9g). There were no obvious P. tricornutum cells adhered to the surfaces in the microscope field (Fig. 9a–d). In this regard, the self-peeling surfaces with an amount of free water may preclude adsorption in proteins and self-refresh changes, and therefore, they are responsible for the low cell adhesion. The difficult to find magnified images (Fig. 9e–g) showed that the Zn/Si-6 surface has fewer adhered diatoms than the Zn/Si-1 surface, although it has a high [N]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [C] molar ratio. This means that there was a significant correlation between the zinc monomer content and the adhesion of diatom cells, even though the hydrolyzed zinc compound was not considered to be the antifouling biocide.
[C] molar ratio. This means that there was a significant correlation between the zinc monomer content and the adhesion of diatom cells, even though the hydrolyzed zinc compound was not considered to be the antifouling biocide.
After seawater exposure, the tri(isopropyl)silyl ester bonds and the Zn-monomer in the top layer of each coating were gradually hydrolyzed into anionic and hydrophilic carboxylic groups to form a thin layer of hydrogel. However, it is important to note here that the Zn/Si-acrylate copolymers have more bubbles with an increase in the Zn-monomer content, which may be caused by the Zn-monomer hydrolysis of the top layer, which makes the polymer chains more hydrophilic (Fig. 9b, c and d). After a sufficient number of bonds are broken, the hydrolyzed copolymer chains, together with the hydrolysable silyl-gels and zinc-compound, are gradually dissolved into the sea water and washed away. In such a way, the next layer of polymer will be exposed to seawater so that the hydrolyzation processes continues to prevent/reduce the settlement and biofouling of marine animals on such a surface coating. This result agreed well with the hydrolysis rate. Therefore, it is vitally important to balance the hydrolysis rate to avoid an increase in surface roughness by varying the hydrolysable monomer content and type.
4. Conclusions
A new type of antifouling Zn/Si-acrylate copolymers composed of tri(isopropyl)silyl acrylate, zinc-2-ethylhexanoate meth-acrylate, ethyl acrylate and methyl methacrylate have been successfully developed by the combination of anti-diatom and self-peeling properties. All of the copolymers exhibited good film forming and erosion properties in artificial seawater, making them suitable as new polymeric binders for hybrid self-polishing coatings. The gradual hydrolytic degradation can effectively slow down the fouling by the diatom. The hybrid self-generating and self-antimicroalgae idea has been conceptually proven in the current study. The newly developed copolymers have better chemical processabilities and can be readily coated onto large objects or objects with complicated geometries by a conventional method such as brushing, spraying or dipping. Moreover, the combination of these two monomers reduces the amount of expensive TIPSA required. This study highlights the developed cost-effective and environmentally friendly antifouling polymer has great potential for industrial applications.Acknowledgements
The work was supported by the International S&T Cooperation Program of China (ISTCP) (No. 2013DFA50480), the Fundamental Research Funds of the Central University (HEUCF20151008) and the National College Students' Innovative Training Program.Notes and references
- M. P. Schultz, Biofouling, 2007, 23, 331–341 CrossRef PubMed.
- A. Lindholdt, K. Dam-Johansen, S. M. Olsen, D. M. Yebra and S. Kiil, J. Coat. Technol. Res., 2015, 12, 415–444 CrossRef CAS.
- D. M. Yebra, S. Kiil and K. Dam-Johansen, Prog. Org. Coat., 2004, 50, 75–104 CrossRef CAS.
- A. C. A. Sousa, M. R. Pastorinho, S. Takahashi and S. Tanabe, Environ. Chem. Lett., 2014, 12, 117–137 CrossRef CAS.
- J. B. Lindén, M. Larsson, B. R. Coad, W. M. Skinner and M. Nydén, RSC Adv., 2014, 4, 25063–25066 RSC.
- C. Bressy, C. Hellio, M. N. Nguyen, B. Tanguy, J. P. Maréchal and A. Margaillan, Prog. Org. Coat., 2014, 77, 665–673 CrossRef CAS.
- F. Hong, L. Y. Xie, C. X. He, J. H. Liu, G. Z. Zhang and C. Wu, Polymer, 2013, 54, 2966–2972 CrossRef CAS.
- D. R. Calabrese, B. Wenning, J. A. Finlay, M. E. Callow, J. A. Callow, D. Fischer and C. K. Ober, Polym. Adv. Technol., 2015, 26, 829–836 CrossRef CAS.
- H. S. Sundaram, Y. Cho, M. D. Dimitriou, J. A. Finlay, G. Cone, S. Williams, D. Handlin, J. Gatto, M. E. Callow, J. A. Callow, E. J. Kramer and C. K. Ober, ACS Appl. Mater. Interfaces, 2011, 3, 3366–3374 CAS.
- E. Martinelli, M. K. Sarvothaman, G. Galli, M. E. Pettitt, M. E. Callow, J. A. Callow, S. L. Conlan, A. S. Clare, A. B. Sugiharto, C. Davies and D. Williams, Biofouling, 2012, 28, 571–582 CrossRef CAS PubMed.
- Y. J. Cho, H. S. Sundaram, C. J. Weinman, M. Y. Paik, M. D. Dimitriou, J. A. Finlay, M. E. Callow, J. A. Callow, E. J. Kramer and C. K. Ober, Macromolecules, 2011, 44, 4783–4792 CrossRef CAS.
- C. J. Huang, N. D. Brault, Y. T. Li, Q. M. Yu and S. Y. Jiang, Adv. Mater., 2012, 24, 1834–1837 CrossRef CAS PubMed.
- L. Mi and S. Y. Jiang, Angew. Chem., Int. Ed., 2014, 53, 1746–1754 CrossRef CAS PubMed.
- F. Fay, I. Linossier, V. Langlois, E. Renard and K. Vallee-Rehel, Biomacromolecules, 2006, 7, 851–857 CrossRef PubMed.
- W. T. Xu, C. F. Ma, J. L. Ma, T. S. Gan and G. Z. Zhang, ACS Appl. Mater. Interfaces, 2014, 6, 4017–4024 CAS.
- L. L. Xue, X. L. Lu, H. Wei, P. Long, J. Xu and Y. F. Zheng, J. Colloid Interface Sci., 2014, 421, 178–183 CrossRef CAS PubMed.
- T. Murosaki, N. Ahmed and J. P. Gong, Sci. Technol. Adv. Mater., 2011, 12, 064706 CrossRef.
- H. P. H. Arp, E. Eek, A. W. Nybakk, T. Glette, T. Møskeland and A. Pettersen, Water Res., 2014, 65, 213–223 CrossRef CAS PubMed.
- Y. Yonehara, H. Yamashita, C. Kawamura and K. Itoh, Prog. Org. Coat., 2001, 42, 150–158 CrossRef CAS.
- P. Durand, A. Margaillan, M. Camail and J. Vernet, Polymer, 1994, 35, 4392–4396 CrossRef CAS.
- S. Mirabedini, S. Pazoki, M. Esfandeh, M. Mohseni and Z. Akbari, Prog. Org. Coat., 2006, 57, 421–429 CrossRef CAS.
- C. Bressy, M. N. Nguyen, B. Tanguy, V. G. Ngo and A. Margaillan, Polym. Degrad. Stab., 2010, 95, 1260–1268 CrossRef CAS.
- C. Bressy, V. G. Ngo and A. Margaillan, Polym. Degrad. Stab., 2013, 98, 115–121 CrossRef CAS.
- F. Hong, L. Xie, C. He, J. Liu, G. Zhang and C. Wu, J. Mater. Chem. B, 2013, 1, 2048–2055 RSC.
- M. N. Nguyen, C. Bressy and A. Margaillan, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 5680–5689 CrossRef CAS.
- C. Bressy, A. Margaillan, F. Faÿ, I. Linossier and K. Rehel, Advances in Marine Antifouling Coatings and Technologies, Woodhead Publishing, Cambridge, UK, 2009, pp. 445–491 Search PubMed.
- M. Lejars, A. Margaillan and C. Bressy, Polym. Chem., 2013, 4, 3282 RSC.
- C. Hellio and D. M. Yebra, Advances in marine antifouling coatings and technologies, Elsevier, 2009 Search PubMed.
- M. Salta, J. A. Wharton, Y. Blache, K. R. Stokes and J. F. Briand, Environ. Microbiol., 2013, 15, 2879–2893 Search PubMed.
- M. Lejars, A. Margaillan and C. Bressy, Chem. Rev., 2012, 112, 4347–4390 CrossRef CAS PubMed.
- R. R. Guillard and J. H. Ryther, Can. J. Microbiol., 1962, 8, 229–239 CrossRef CAS PubMed.
- M. Ferriol, A. Gentilhomme, M. Cochez, N. Oget and J. Mieloszynski, Polym. Degrad. Stab., 2003, 79, 271–281 CrossRef CAS.
- M. N. Nguyen, C. Bressy and A. Margaillan, Polymer, 2009, 50, 3086–3094 CrossRef CAS.
- X. F. Sun, S. G. Wang, X. M. Zhang, J. Paul Chen, X.-M. Li, B.-Y. Gao and Y. Ma, J. Colloid Interface Sci., 2009, 335, 11–17 CrossRef CAS PubMed.
- G. Beamson and D. Briggs, High Resolution XPS for Organic Polymers: The Scienta ESCA300 Database, John Wiley & Sons, New York, 1992, pp. 128–131 Search PubMed.
- W. Yu, E. T. Kang, K. G. Neoh and S. Zhu, J. Phys. Chem. B, 2003, 107, 10198–10205 CrossRef CAS.
- P. J. Molino and R. Wetherbee, Biofouling, 2008, 24, 365–379 CrossRef CAS PubMed.
- S. K. Kawakami, M. Gledhill and E. P. Achterberg, Biometals, 2006, 19, 51–60 CrossRef CAS PubMed.
- W. J. Yang, T. Cai, K.-G. Neoh, E.-T. Kang, G. H. Dickinson, S. L.-M. Teo and D. Rittschof, Langmuir, 2011, 27, 7065–7076 CrossRef CAS PubMed.
- D. Pranantyo, L. Q. Xu, K. G. Neoh, E. T. Kang, W. Yang and S. L. M. Teo, J. Mater. Chem. B, 2014, 2, 398–408 RSC.
- B. Tesson, M. J. Genet, V. Fernandez, S. Degand, P. G. Rouxhet and V. Martin-Jézéquel, ChemBioChem, 2009, 10, 2011–2024 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra24270c |
| This journal is © The Royal Society of Chemistry 2016 |