Use of feedback system control in optimizing chemical combinations to synthesize nanoparticles with desired characteristics†
Abstract
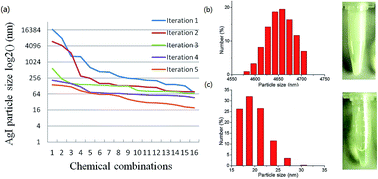
Nanoparticles (NPs) have found extensive applications in biological diagnosis as well as new energy and chemical devices. One of the major routes to fabricate NPs is chemical synthesis under specifically controlled conditions, when surfactants are often added during or immediately after precipitation. However, interactions between multiple surfactants and reactants could be extremely complicated. Therefore, identifying the optimal formula of multiple chemicals to synthesize NPs with desired characteristics is a daunting task. In this study, we applied an experimental feedback system control (FSC) method and rapidly identified optimal chemical combinations that synthesize silver iodide (AgI) NPs with a target size of 10 nm by only testing less than 0.5% of the total possible chemical combinations. The FSC approach allows rapid optimization of combinatorial chemicals without the requirement of a complete understanding of the underlying chemical interactions. The method introduced here, with slight modification, could be easily applied for other NPs synthesis scenarios.

 Please wait while we load your content...
Please wait while we load your content...