Photoluminescence study of oleic acid capped and hexanoic acid washed CdS quantum dots
Abstract
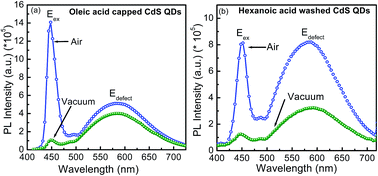
The effects of oxygenic versus oxygen-free environment on the excitonic and defect emission of colloidal oleic acid (OA) capped and hexanoic acid washed CdS quantum dots (QDs) were studied using continuous photoluminescence (PL) measurements. In vacuum (∼10−3 mbar) OA capped CdS show 90% and 30% quenching of excitonic and defect emission intensity, respectively, whereas acid washed samples show 80% excitonic and 78% defect emission intensity quenching. It is also observed that the excitonic and defect emission intensities are fully recovered in air on cycling the air/vacuum pressure cycles. Our analysis suggests that due to high non-radiative recombination, both excitonic as well as defect emission intensity quenches. The low temperature PL study clearly shows that the enhancement in defect emission intensity for acid washed QDs is about three times higher than that of OA capped QDs, which is due to suppression of non-radiative recombination. Our work shows that emission can be controlled by modulating the degree of passivation ether by acid washing process or controlling the air/vacuum environment or both.

 Please wait while we load your content...
Please wait while we load your content...