Catalytic oxidation of nitric oxide (NO) with carbonaceous materials
Yafei Shen*,
Xinlei Ge and
Mindong Chen
Jiangsu Engineering and Technology Research Center of Environmental Cleaning Materials (ECM), Jiangsu Key Laboratory of Atmospheric Environment Monitoring and Pollution Control (AEMPC), Collaborative Innovation Center of Atmospheric Environment and Equipment Technology (AEET), School of Environmental Science and Engineering, Nanjing University of Information Science & Technology, Nanjing 210044, China. E-mail: yafeisjtu@gmail.com; 376754097@qq.com
First published on 12th January 2016
Abstract
The catalytic oxidation of NO to NO2 at ambient temperatures has been a promising route for controlling NO emissions, since NO2 is subsequently removed as nitric acid in the presence of water. Because of their large surface area, high porosity, and relative chemical inertness, carbon-based materials are very attractive in de-nitrification (De-NOx) as catalysts or catalyst supports. This paper reviewed the catalytic oxidation of NO to NO2 over commonly-used carbon materials including activated carbons (ACs), activated carbon fibers (ACFs) and carbon xerogels (CXs). The NO conversion is often influenced by the surface characteristics of carbon materials (e.g., pore structure, surface areas, functional groups, and morphology), O2 concentration, and reaction temperature. With the addition of metal actives, the catalytic performance could be significantly improved. Catalytic reaction and adsorption are two key points. Further, the strong dependence of NO conversion on the O2 concentration concludes that O2 is first adsorbed on the carbon surface, and then it reacts with NO to form adsorbed NO2, which desorbs to the gas phase. Considering the economic efficiency, carbon precursors from biomasses could be fabricated into the desired carbonaceous materials by means of functionalization. In addition, the integrated strategy of desulfurization (De-SOx) and De-NOx could be developed by carbon materials with the proper modification methods.
1. Introduction
Atmospheric contamination is one of the most significant environmental problems caused by the progressive industrialization of the planet.1 Nitrogen oxides (NOx) commonly derived from the combustion of fossil fuels or solid wastes are considered primary atmospheric pollutants, being responsible for a wide range of environmental problems such as photochemical smog, acid rain, tropospheric ozone and ozone layer depletion.2 Furthermore, they cause serious health problems in humans.3 Hence, many great efforts have been made to develop technologies for flue gas purification. As for NOx removal, advanced flue gas treatment technologies are adopted, including dry and wet techniques. The dry techniques could be classified as selective catalytic reduction (SCR) by NH3 at 300–500 °C,4 selective non-catalytic reduction (SNCR),5 adsorption6 and electron beam irradiation.7 The wet techniques use scrubber columns in which NOx is absorbed by absorbents.The most popular NOx abatement technology is SCR with ammonia (NH3), but this process has several obvious drawbacks, such as high reaction temperatures (>300 °C) and un-reacted reducing agents.8 Besides, typical NOx emissions from combustion processes contain significantly more NO than NO2, and the inclusion of NO oxidation step prior to the SCR process is useful for increasing SCR rates of reaction.9 Thus, the catalytic oxidation of NO to NO2 at ambient temperatures has been a promising route for controlling NO emissions, since NO2 is subsequently removed as nitric acid in the presence of water.10–13 The NO2 absorption efficiencies can reach as high as 100% in water or basic solutions.14–17 Consequently, oxidation of NO into NO2 at lower temperatures (e.g., room temperature) has been a prospective high-efficiency step for the removal of NO. In particular, it is practicable to remove NO at ambient temperature in the presence of water vapor, thus much attention has been paid to the oxidation of NO into NO2.18–20
NO oxidation is thermodynamically favorable below 200 °C, but kinetically limited.21 Oxidation strategies consist of homogeneous catalysis with oxidizing additives, and heterogeneous catalysis with unsupported metal oxides or supported catalysts.21–23 Homogeneous gas-phase NO oxidation is unusual in that the reaction rate increases with the decrease of temperature from 273 to 600 K, resulting in a small but negative activation energy.24 These techniques are generally effective, but the costs resulting from the use and potential release of hazardous chemicals, catalyst deactivation due to SO2 poisoning, and thermodynamic limitations associated with necessarily high temperatures inhibit their application. Low temperature oxidation of NO coupled with absorption of NO2 is an alternative NOx abatement strategy. Such a strategy could avoid expenses associated with SCR (e.g., catalysts, gas reheating) and the threat of ammonia slip.
2. Carbon-catalyzed oxidation
Carbon materials have been regarded as good catalysts for NO oxidation to NO2.25 The surface chemistry of carbon materials often influences their adsorption, catalytic, acid–base and redox properties, as well as their hydrophilic or hydrophobic character. Hydrogen, oxygen, sulfur, nitrogen, and other elements could also be found on the activated carbon surface.Catalytic oxidation of NO (2NO + O2 → 2NO2) over microporous activated carbons combined with subsequent absorption process of NO2 as a more soluble NOx specie is an alternative to SCR. Compared with SCR, carbon catalyzed NO oxidation operates at low temperatures (<100 °C) and could potentially be used for simultaneous control of multiple pollutants.26 While combination of NO oxidation with NO2 absorption, a thorough understanding of the carbon-catalyzed NO oxidation is also important for improving NO reduction via fast SCR reactions.27,28 Since Mochida's initial studies introducing the carbon-catalyzed NO oxidation, extensive efforts have been taken on understanding the reaction mechanism. After twenty years, however, the mechanism continues to be debated.29–32 The complexity of activated carbon (i.e., variability in the physical and chemical properties of activated carbon) and NO auto-oxidation33 make characterizing the NO oxidation mechanism challenging. The investigation of chemical functionalities on NO oxidation showed that oxygen functional groups can impact NO2 adsorption capacity and transient oxidation kinetics.34–36
The kinetics of heterogeneous catalysis mainly depends on: (1) reactant adsorption, (2) catalytic reaction at active sites, and (3) product desorption. The reaction rate, therefore, is influenced by the physical and chemical properties of the catalysts.37 Proposed mechanisms for carbon-catalyzed NO oxidation are summarized as follows. Mochida29–31 first investigated the influence of physical and chemical properties of carbon, as well as processing parameters (e.g., NO and O2 concentrations, reaction temperature, and gas hourly space velocity), on steady-state NO oxidation kinetics. Mochida et al.30 suggested that [NO–O–NO2]ad is a crucial intermediate for NO2 formation/desorption and that NO adsorption is the rate-determining step due to competition with desorbed NO2 and intermediates. This mechanism, consistent with observed kinetic profiles, suggests that (NO)2, NO2, and (NO2)2 are not necessary as reaction intermediates.38 Then, Adapa et al.32 proposed Langmuir–Hinshelwood (L–H) and Eley–Rideal (E–R) mechanisms for catalyzed NO oxidation. Herein, in the L–H mechanism, dissociated oxygen activated by carbon reacts with adsorbed NO. In the E–R mechanism, gaseous O2 can directly react with NO adsorbed in micropores. Both mechanisms rely on the evolution of similar reactive intermediates, including C*–NO, C*–NO2, C*–NO3, and C*–NO–NO3.
The mechanism of catalytic NO oxidation over carbonaceous materials is a complex process, since it involves multiple steps, including carbon gasification, oxidation of NO and the release of NO2. Some reaction pathways have been proposed to explain the oxidation of NO into NO2. Ahmed et al.39 suggested that NO is oxidized by oxygen to NO2 in the gas phase and then NO2 is adsorbed on the carbon surface. However, the homogeneous oxidation of NO in the gas phase is too slow to account for the conversions observed with the activated carbon. Mochida et al.40 proposed that NO is adsorbed, oxidized into adsorbed NO2, which desorbs as NO2. Since NO is a supercritical gas at ambient temperature, very little NO can be physically adsorbed.
Claudino et al.41 proposed that NO oxidation on the activated carbon is a micropore filling process with NO as the adsorbed species. Namely, the narrow micropores in activated carbon act as catalytic nanoreactors for NO oxidation. It is well known that narrow micropores are very important for gas adsorption.42–44 Rathore et al.45 defended that the gaseous NO and O2 adsorb on the active sites of activated carbon fibers, followed by NO oxidation into adsorbed NO2. The adsorbed NO2 further reacts and forms various intermediates in the adsorbed phase, such as NO3 and NO–NO3. Finally, the NO–NO3 adsorbed intermediate is desorbed into NO2. It has also been proposed that NO reacts with the surface oxygen atoms present on activated carbons.46,47 However, this argument is difficult to reconcile with the literature, which refers that pre-adsorption of O2 on carbon materials inhibits the removal of NO.39,48,49 It is suggested that NO from the gas phase reacts with chemisorbed oxygen, forming the chemisorbed NO2.48 The improvement of the catalytic performance of nitrogen-containing carbons in NO oxidation can be explained from the point of view of the electronic theory of catalysis. The extra π-electrons of nitrogen groups could facilitate the formation of NO2 caused by molecular oxygen activation.50 Activated carbons treated with nitric acid as well as with melamine are the most active for NO oxidation.51 The catalyst bed is saturated with NO2 resulting in a large increase in the NO outlet concentration. Meanwhile, breakthrough of NO2 occurs, which results in vacant sites. Adsorption of O2 on the new vacant sites may proceed only when NO2 is desorbed to release these sites. Therefore, NO2 desorption is the rate-limiting step.32
In addition, Zhang34,52 proposed that NO2 formed via the NO catalytic oxidation, decomposes and causes rapid oxidation of the carbon surface with subsequent NO2 chemisorption. Furthermore, this proposed concept was extended by identifying and quantifying these generated oxygen groups, highlighting their contribution toward the acidity of carbon-based catalyst and describing their impact on transient NO oxidation kinetics.36 In theory, all proposed NO oxidation mechanisms stress the significance of catalytic sites of carbon, the number of which should be proportional to accessible surface area of carbon. However, steady-state NO oxidation kinetics are independent of the accessible surface area of carbon.31 Steady-state NO conversion efficiency increases with the increase of the NO concentration (i.e., [NO]). This Langmuir-type dependence on [NO] supports that the reaction is not limited by the availability of active sites. This is the opposite of SCR systems53 and metal oxide catalyzed NO oxidation, where the steady-state NO conversion efficiency is inversely proportional to [NO].54 Further investigation into the carbon-catalyzed NO oxidation is necessary to address the role of the physical and chemical properties of carbon on transient and steady-state oxidation kinetics.
Recently, Zhang et al.55 studied an updated mechanism of NO oxidation catalyzed by microporous activated carbon fiber cloth. The updated NO oxidation mechanism mainly consists of two consecutive steps: (1) NO2 is rapidly formed through gas phase reactions between NO and O2 in carbon micropores; formation and decomposition of C*–NOx species,40 does not take place, and (2) newly formed NO2 adsorbs to the carbon surface is associated with NO2 reduction and development of C*–NOx and C*–O functionalities. The physical properties of activated carbon control steady-state NO oxidation kinetics with chemical properties of carbon having no apparent impact. Additionally, carbon's adsorption and surface reaction tendencies impact transient conversions by allowing for destruction of the NO2 product and regeneration of the NO reactant. The second mechanistic step diminishes as the carbon surface saturates with adsorbed NOx species and oxygen functionalities. At steady-state, the carbon surface is saturated, preventing the further NO2 reduction. In summary, the first step of the mechanism (NO oxidation in micropores) controls steady state NO oxidation kinetics, while the second step (NO/carbon surface reactions) controls transient NO oxidation kinetics.
3. Carbon-based catalysts
Carbon-based materials including activated carbons, activated carbon fibers are very attractive in de-nitrification (De-NOx) as catalysts or catalyst supports.56,57 Most researches involved in the De-NOx over carbon-based materials primarily focused on the removal or conversion of high concentration NO in flue gas,58,59 and carbon materials were used as catalyst supports.Porous materials of carbon molecular sieves are disordered forms of graphitic carbon produced from the pyrolysis and activation of organic materials at high temperatures.60 These carbon materials have important applications in gas adsorption, catalysis, and phase separation.61 Their porous structures typically contain combinations of micropores (<20 Å), mesopores (20–500 Å), and macropores (>500 Å).60 And these materials exhibit adsorption volumes and specific surface areas up to 0.5–0.8 cm3 g−1 and 700–1800 m2 g−1, respectively.62 Loiland et al.63 studied the porous carbon molecular sieves for the NO oxidation based on the results of Artioli et al.,64 which suggested that many microporous solids could provide increased reactivity compared to the homogeneous phase reaction.
3.1. Activated carbon (AC)
ACs have been widely used as adsorbents of gases and vapors, catalyst supports, and separation media.65,66 Their features for pollutant removal are large surface area, rich microporosity,67 and rapid adsorption velocity.68–71 AC desulfurizer can be used for gas cleaning and H2S removal by sorption enhanced catalytic oxidation at low temperatures.72 Guo25 studied the NO oxidation by the commercialized ACs, such as coconut-AC, pitch-ACF, polyacrylonitrile-ACF (i.e., PAN-ACF and PAN-ACFKOH) using the dry gas of NO–O2–N2 at 30 °C. The steady-state conversion of NO to NO2 mainly depends on the O2 concentration, the temperature and the properties of AC.NO conversion with various ACs versus the O2 concentration is shown in Fig. 1. The oxidation of NO to NO2 obviously increased with the increase of O2 concentration with ACF. However, the dependence is not obvious with the coconut-AC. Even in the presence of 2% O2, the NO conversion can reach 83% by AC. The pitch-ACF showed the higher activity than the PAN-ACF for the oxidative removal of NO. In the same gas space flow rate of 1500 h−1, the order of activity is PAN-ACF < pitch-ACF < coconut-AC. Interestingly, the coconut-AC shows very high activity for the oxidation of NO to NO2 even with the low concentration of O2. This suggests that oxidative removal of NO by ACs could be a compatible process for industry. In addition, ACs have higher strength and lower cost than ACFs.25
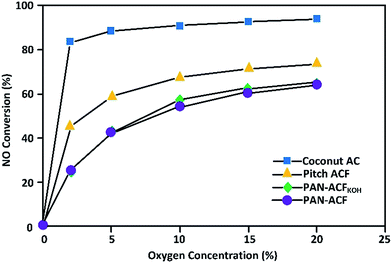 | ||
| Fig. 1 NO conversion in steady state versus O2 concentration by different ACs at 30 °C for the dry gas with 400 ppm NO and space flow rate 1500 h−1 (reproduced from ref. 25 with permission from Elsevier). | ||
The overall oxidation reaction of NO to NO2 in a dry gas is expressed as: NO + ½O2 = NO2. And the oxidation reaction rate of NO in a dry gas can be given by the following kinetic equation:
| r = kPNOαPO2β. |
| R = kcPNOPO2β(F/W) |
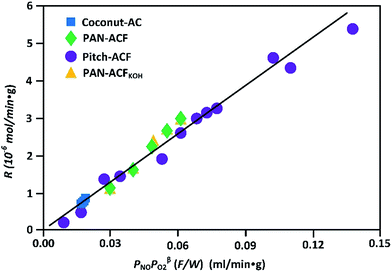 | ||
| Fig. 2 Apparent reaction rate versus PNOPO2β(F/W) of NO oxidation in dry gas on ACs at 30 °C (reproduced from ref. 25 with permission from Elsevier). | ||
Recently, there has been an increasing interest for N-doped carbon materials.73–78 The presence of nitrogen atoms in the carbon matrix could enhance the catalytic activity of carbon materials in oxidation reactions and increase the ability to adsorb acidic gases.79,80 The effect of nitrogen doping of carbons on catalytic properties could be attributed to two overlapping effects, catalysis by basic surface sites and by electron donation.81 Nitrogen containing functionalities confer basic properties to the surface of AC, which enhance the interaction with acid molecules;77 moreover, the extra electrons can be transferred to adsorbed species.82
The nature and concentration of functional groups present on the surface of AC mainly depend on the activation method in the synthesis, but they can be modified by thermochemical treatments.83 Nitrogen groups can be often introduced to the structure of AC by treatments with urea in the liquid phase or with ammonia in the gas phase at high temperatures.84,85 Sousa et al.18,51 modified the AC with a high density of surface nitrogen Lewis basic sites for NO oxidation. The incorporation of nitrogen can significantly improve the catalytic activity of the modified AC. The NO conversion increased with the nitrogen content is associated with electron transfer from the carbon surface to the NO molecules. Stöhr et al.84 found that ammonia treatment of the carbon surface can facilitate the chemisorption of molecular oxygen. According to them, when carbon atoms are substituted by nitrogen atoms within the graphene layers, the extra electrons can be easily transferred to the adsorbed species, forming reactive surface intermediates. The highest amount of NO oxidized at room temperature was achieved with the highest amount of nitrogen containing groups.86
O2 concentration can strongly influence the NO oxidation over carbon materials. O2 is first chemisorbed on the surface (eqn (1)) and then NO reacts with surface O2 to form NO2 adsorbed on the carbon surface (eqn (2)). O2 favours the formation of new active sites on the surface of carbon material for NO adsorption. Formation of a NO dimer (NO)2 has been proposed as a possible mechanism to explain the reduction of NO to N2 at low temperatures (eqn (3)). The formation of (NO)2 occurs as the concentration of strongly adsorbed NO is high and there is a higher probability that two NO molecules could be located adjacently to form a (NO)2 dimer.
| 2Cf + O2 → 2C–O | (1) |
| C–O + NO → C–NO2 | (2) |
| (NO)2 + 2Cf → N2 + Cf–O | (3) |
The formation of N–N and C–O bonds, followed by splitting of N–O bonds to produce a N2 molecule. These reactions are exothermal, which means that they are favored at lower temperatures. To assess the influence of the nitrogen groups, the amount of NO converted, normalized by the surface area, was plotted against the nitrogen content of the AC as shown in Fig. 3. It can be indicated that the presence of nitrogen groups enhances the oxidation of NO to NO2. The extra electrons resulting from the substitution of C for N in the aromatic ring are delocalized and can be transferred to absorbing species to form reactive surface intermediates.87
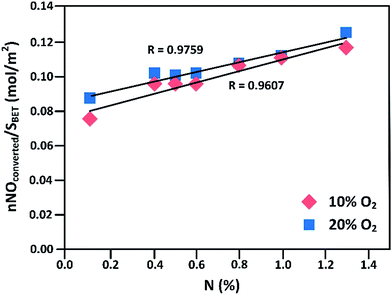 | ||
| Fig. 3 Amount of NO converted per unit surface area versus nitrogen content of the AC (reproduced from ref. 18 with permission from Elsevier). | ||
3.2. Activated carbon fiber (ACF)
ACFs have been widely used as adsorbents and catalysts supports due to their micro porous texture having large surface area, significant adsorption characteristics for a number of air pollutant species, and ability of regeneration with ease. Besides, ACFs have flexibility to be applied in reactors, where they can be wrapped or rolled easily.32 Two possible states of O2 can be considered, either dissociately adsorbed on the surface or molecular in the gaseous phase, in the mechanism for the oxidation of NO by ACF. Table 1 presents the two possible mechanisms of NO oxidation over carbon catalysts. As for the Langmuir–Hinshelwood model (mechanism 1), gaseous NO and O2 are assumed to adsorb on the vacant active sites of ACF, followed by oxidation of NO into adsorbed NO2. Subsequently, the adsorbed NO2 reacts and forms various intermediates such as, NO3 and NO–NO3 in the adsorbed phase. Finally, the adsorbed intermediate NO–NO3 is desorbed into NO2 from the surface, thereby releasing the vacant sites for adsorption of successive molecules of adsorbates. In accordance with the Eley–Rideal model (mechanism 2), adsorbed NO could be assumed to react with the gaseous O2 (instead of adsorbed O2) and yield the adsorbed NO2. The subsequent reactions of adsorbed NO2 are proposed to proceed similar to those outlined for mechanism 1.| Mechanism 1 | Mechanism 2 |
|---|---|
| NO + Cf ↔ C–NO (a) | NO + Cf ↔ C–NO (a) |
| O2 + 2Cf ↔ 2C–O (b) | 2C–NO + O2 ↔ 2C–NO2 (b) |
| C–NO + C–O ↔ C–NO2 + Cf (c) | C–NO2 + C–NO2 ↔ C–NO3 + NO + Cf (c) |
| C–NO2 + C–NO2 ↔ C–NO3 + NO + Cf (d) | C–NO3 + C–NO ↔ C–NO–NO3 + Cf (d) |
| C–NO3 + C–NO ↔ C–NO–NO3 + Cf (e) | C–NO–NO3 → 2NO2 + Cf (e) |
| C–NO–NO3 → 2NO2 + Cf (f) |
Zhang et al.36,55 studied the mechanism and kinetic of NO oxidation by ACF cloth. To date, most carbon-catalyzed NO oxidation studies aim to maximize steady-state NO conversion rates. There has been limited emphasis on accelerating the path to achieving steady-state conversions, which involves at least co-adsorption of NO and O2, catalytic reaction, and subsequent desorption of formed NO2.40 To improve this facet of the carbon catalysts, studies describing the interfacial catalytic reactions between NOx and carbon are required. The impact of surface functional groups (e.g., nitrogen, oxygen) on AC has been studied the interactions between NOx and carbon.18,48,88 Oxygen functionalities are retained on carbon due to the decomposition of formed NO2 intermediates over the reducing carbon surface.79 The formation of oxygen groups can impact NO/NO2 adsorption/desorption kinetics, accelerating the release of NO2 from the carbon surface. In Atkinson's work,36 the acidic oxygen functional groups on ACF cloth are developed for NO oxidation. Carbon catalysts with acidic oxygen functionalities has been considered as promising NO oxidation catalysts, as confirmed with NO2 and nitric acid treatments. In general, chemical properties of carbon materials impact NO oxidation kinetics, while physical properties impact the steady-state NO oxidation rate.
Wang et al.89 verified that NO can be catalytically oxidized into NO2 over activated carbon nanofibers (ACNFs). After further graphitized at high temperature, graphitized porous carbon nanofibers (GPNF) is formed. The catalytic efficiency of NO oxidation could be enhanced remarkably at ambient temperature.90 The morphology of the ACNFs is shown in Fig. 4. The average fiber diameter of ACNF without graphitization is in the range of 200–300 nm, and the surface is clean and smooth (Fig. 4A). After graphitization, the fiber diameter shrank visibly due to the burn-off and ordering of graphite layers at higher temperatures of 1900 °C and 2400 °C (Fig. 4B and C). Moreover, the surface of GPNFs became rough with some wrinkle and small holes.
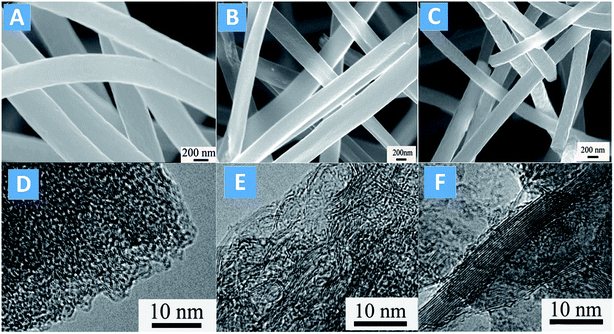 | ||
| Fig. 4 SEM images of ACNF (A), GPNF-1900 (B) and GPNF-2400 (C); TEM images of ACNF (D), GPNF-1900 (E) and GPNF-2400 (F) (reproduced from ref. 90 with permission from Elsevier). | ||
TEM analysis can provide more insights into the stacking of graphite layers, and the crystallographic changes in ACNFs can be directly acquired. As shown in Fig. 4D–F, compared with the non-graphitized ACNF, great changes occurred in the inner layer where the graphite sheets became stiff and amorphous carbon layer in outer-layer became thinner. With the increase of graphitization temperature, the graphite sheets in inner layer were arranged in order and more continuous, and the orientation of disorganized hexagonal surface of crystallite became ordered. Interestingly, some new defects appeared in the surface and end of the graphite sheets. The defects in ACNFs are derived from the dangling bonds, which are saturated with oxygen functionalities. However, graphitization can remove those functionalities to form new defects at the surface of fibers. Due to the high degree of graphitization and defects, GPNFs can provide more active sites for the catalytic oxidation of NO transformation into NO2. In particular, the NO oxidation ratio for ACNFs, GPNF-1900 and GPNF-2400 was 11%, 38%, 45%, respectively, revealing that the GPNFs could be used as promising catalysts in catalytic oxidation of NO at room temperature.
The graphitic CNFs (pyrolytically stripped) were found to be the most active in the catalytic process followed by heat treatment from 1500 to 3000 °C.91 For example, it was proved that activated polyacrylonitrile carbon nanofibers (PCNFs) to determine effect of high temperature treatment on their catalytic activity during the oxidation of NO to NO2.90 The oxidation conversion could be significantly improved by using graphitized PCNFs, but this method always requires higher temperatures92,93 that is not economical due to energy consumption; therefore it is necessary to prepare CNFs with similar structure that can be formed at low temperatures.
Graphene is a commercially attractive material that possesses a large theoretical specific surface area,94–98 a high Young's modulus, good thermal conductivity and good electrical conductivity that could be used for a variety of potential applications including in transparent conductive electrodes.99,100 More recently, Guo et al.101 prepared microdomain-graphitized polyacrylonitrile (PAN) nanofibers by adding the graphene oxide (GO) into the precursor via the electrospinning method. These electrospun nanofibers were stabilized in ambient atmosphere, carbonized in N2 and treated in NH3 atmosphere for NO oxidation with a low concentration (50 ppm) at room temperature. The GO nanosheets can be embedded in the electrospun fibers and converted to reduced graphene oxide (rGO) by heat treatment (namely PGCNFs as illustrated in Fig. 5J). A series of rGO content was embedded in the PCNFs forming a carbon–carbon hybrid material that showed a microdomain-graphitized and porous structure. SEM images of the PCNF and PGCNF samples are shown in Fig. 5A–F. The samples experienced instantaneous cyclization, dehydrogenation and cross-linking reactions in the presence of O2, thereby creating nanofibers that are infusible at higher temperatures.102,103 The PCNFs are homogeneous, and smooth with average diameters of approximately 200 nm. With the addition of GO, the surface of the PGCNFs becomes coarser. Furthermore, large amounts of tiny rGO fragments are embedded inside the PCNFs and are not externally visible. However, the large-scale GO sheets with lateral dimensions of 0.5–1.0 μm cannot be enclosed completely by the PCNFs resulting in the presence of some “rose-like” nodes on the surfaces of the materials. The rGO sheets provide catalytic active sites embedded inside the PCNFs. In addition, the nitrogen-containing functional groups can play important roles on the enhancement of the catalytic oxidation of NO to NO2. As shown in Fig. 5G–I, the samples with 5 wt% GO exhibit the most catalytic oxidation of NO into NO2.
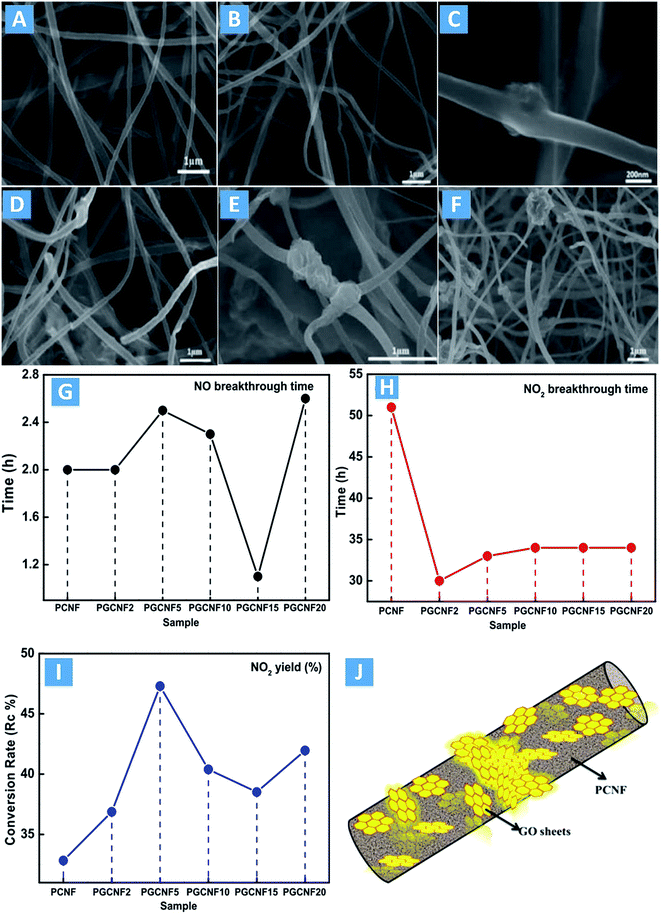 | ||
| Fig. 5 SEM images of PCNF and PGCNF samples: (A) PCNF, (B) PGCNF2, (C) PGCNF5, (D) PGCNF10, (E) PGCNF15, and (F) PGCNF20. Variations in (G) NO breakthrough time, (H) NO2 breakthrough time and (I) NO to NO2 conversion rate of the PCNF and PGCNF samples. The numerals following PGCNF reflect the wt% of GO incorporation. (J) Schematic of a microdomain-graphitized PCNF (reproduced from ref. 101 with permission from Elsevier). | ||
The superiority of CNFs over ACFs in catalytic and adsorption applications lies in the higher stability of CNFs in acidic/basic media and chemical activity.104–107 ACF- or CNF-supported metal catalysts can be also used in the abatement of NO emissions by oxidation or reduction.89,90,108 Talukdar et al.109 developed the CeO2 and Cu nanoparticles dispersed in CNFs/ACFs for the removal of NO by catalytic oxidation at room temperature. The CNFs/ACFs were prepared by means of growing CNFs on an ACF substrate via catalytic chemical vapor deposition (CVD). Subsequently, CeO2 and Cu nanoparticles could be in situ incorporated into the ACFs prior to CVD. The Cu nanoparticles can therefore play dual roles: (1) catalyzing the growth of CNFs and (2) catalyzing the oxidation of NO to NO2. CeO2 had a promotional effect on the catalytic activity of Cu through the release of nascent oxygen during the redox cycle and a synergistic interaction with the Cu nanoparticles.
Oxygen is dissociatively adsorbed on the vacant sites of ACFs. It reacts with the NO adsorbed on the adjacent sites to produce NO2. Then, the adsorbed NO undergoes transformations to produce intermediate surface complex and NO2, leaving behind the active sites for successive adsorption. The redox reaction involving the synergistic interaction between Ce3+ and Cu2+ produces lattice oxygen and restores the oxidation state (4+) of Ce in CeO2. Fig. 6 schematically illustrates adsorption/desorption of the reacting species and the synergistic interaction between CeO2 and Cu nanoparticles. The proposed mechanism for the reaction, 2NO + O2 → 2NO2 is as follows.
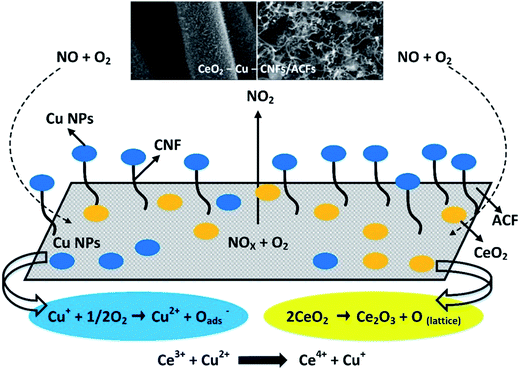 | ||
| Fig. 6 Schematic diagram of the adsorption–desorption reaction and synergistic interaction between CeO2 and Cu (reproduced from ref. 109 with permission from the American Chemistry Society). | ||
The catalytic oxidation of NO over CeO2–Cu-CNFs/ACFs consists of two simultaneous steps. Step A involves the adsorption–desorption of NO and O2 on the CNFs/ACFs and catalytic oxidation of NO to NO2.
| NO + X ⇌ NO–X | (4) |
| O2 + 2X ⇌ 2O–X | (5) |
| NO–X + O–X ⇌ NO2–X + X | (6) |
| 2NO2–X ⇌ NO3–X + NO + X | (7) |
| NO3–X + NO–X ⇌ NO3–NO–X + X | (8) |
| NO3–NO–X → 2NO2+ X | (9) |
Step B involves the release of nascent oxygen and synergistic interaction between CeO2 and Cu nanoparticles.
| 2CeO2 → Ce2O3 + O(lattice) | (10) |
| Ce3+ + Cu2+ → Ce4+ + Cu+ | (11) |
| Cu+ + ½O2 → Cu2+ + O(adsorbed)− | (12) |
Fig. 7 presents the comparative performances of the ACFs/CNFs based materials for the NO oxidation. The catalytic performances were found to be in the following order: CeO2–Cu-CNFs/ACFs > Cu-CNFs/ACFs > Cu-ACFs > CeO2-ACFs > ACFs. The synergistic interaction between the Cu nanoparticles and CeO2 enhanced the oxidation rate. The maximum NO conversion using the CeO2–Cu-CNFs/ACFs developed was 80% for a 500 ppm NO concentration at room temperature (30 °C). Generally, loading some specific metal actives could enhance the catalytic performance for NO conversion. However, the synergistic interaction is considerable for the preparation of metal loaded carbon catalysts.
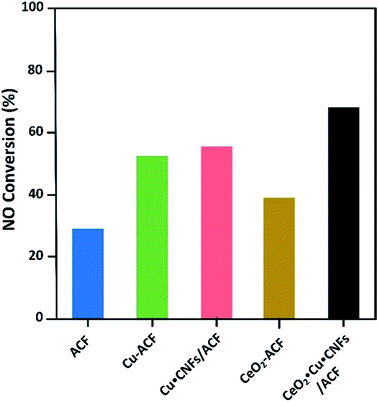 | ||
| Fig. 7 Comparative performance of the prepared materials in the oxidation of NO (T = 30 °C, P = 1 bar, W = 1 g, NO = 1000 ppm, Q = 37.5 sccm, O2 = 20%) (reproduced from ref. 109 with permission from the American Chemistry Society). | ||
3.3. Carbon xerogel (CX)
CXs have been considered as novel porous carbon materials that can be obtained from carbonization of organic xerogels prepared by sol–gel polycondensation of some specific monomers, such as resorcinol and formaldehyde, following Pekala's method.110 A polycondensation reaction can occur between resorcinol and formaldehyde, yielding a three-dimensional polymer matrix, the RF hydrogel. After solvent exchange and drying, followed by carbonization, CX is obtained. Subsequent activation can be used for modification of the surface properties of the material.111 In addition, CXs can be prepared with other monomer combinations, such as melamine/formaldehyde/resorcinol, phenol/furfural, urea/formaldehyde/resorcinol or polyurethane.112 If nitrogen containing precursors (i.e., melamine and urea) are used, CXs enriched with non-reactive nitrogen located in the graphene sheets can be obtained as well.113,114Sousa et al.48,115 studied the CXs with or without nitrogen-doped treatment for the catalytic oxidation of NO. The strong dependence of NO conversion on the O2 concentration results in the conclusion that O2 is first adsorbed on the surface of CXs, and then it reacts with NO to form adsorbed NO2. Finally, NO2 can desorb to the gas phase (as illustrated in Fig. 8A). Fig. 8B shows the effect of O2 concentration on NO conversion at 25 °C. In the presence of O2, the carbon materials catalytically oxidize NO with rates larger than those of the homogeneous oxidation (36% for 20% O2). The oxidation of NO to NO2 increases with the increase of O2 concentration from 2% to 10%. Further increase of O2 concentration from 10% to 20% does not affect so much the conversion. Even in the presence of 2% O2, the NO conversion can reach 86% with sample CX-5.3-900 °C. The maximum NO conversion can be obtained when 10% of O2 was used. Above the optimum O2 concentration, the NO conversion levels off ascribed to saturation of the adsorption sites with atomic oxygen. The highest NO conversion was obtained on CX-5.3-900 °C with 10% of O2 (98%). The CXs showed a high stability for the NO oxidation. With 1000 ppm of NO and 10% of O2 over the most efficient catalyst (namely CX-5.3-900 °C), a steady-state conversion is reached after 26 h of reaction (98%), and does not change thereafter (Fig. 8C).115 The removal of NO by the catalytic oxidation of NO to NO2 on CXs is feasible at relatively low temperatures. Generally, NO conversion increases with the O2 concentration in the gas phase. The highest NO conversions were obtained by the samples with the highest specific surface areas. In a steady state, the micropores are occupied with NO2 adsorbed, so only the mesopore surface area is available for reaction.
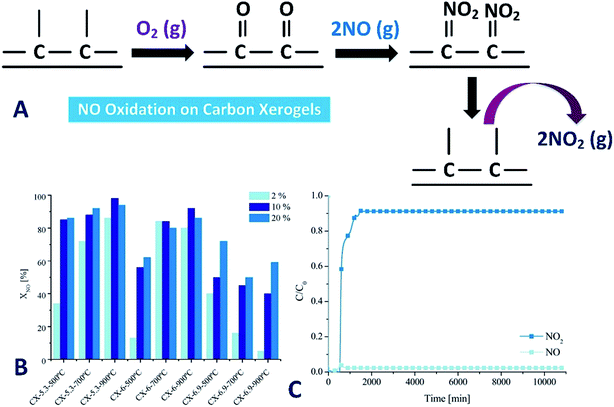 | ||
| Fig. 8 (A) Simplified scheme of NO oxidation on CXs; (B) effect of O2 concentration on NO conversion at 25 °C; (C) evolution of profiles of NO and NO2 during one week of reaction with sample CX-5.3-900 °C (reproduced from ref. 115 with permission from the MDPI). | ||
3.4. Other carbon materials
Carbon plays a dual role as a catalyst or a catalyst support due to its large specific surface area, high porosity, and relative chemical inertness. Advantageously, carbon materials can be prepared from biomass, an attractive property for decreasing the so-called “carbon-footprint” of a biomass transformation process. Carbon could be chemically functionalized and/or decorated with metallic nanoparticles and enzymes to impart or improve novel catalytic activity.116 Furthermore, the development of multifunctional catalysts, possibly originated from more emerging carbon materials such as graphene, carbon nanotubes, and carbon monoliths is also required for deep theoretical study of NO oxidation. However, a relative economical carbon catalyst is urgently developed for industrialization. In addition, carbon precursors derived from residual biomasses could be fabricated into the desired carbonaceous materials by functionalization.117Biochar is a by-product from thermal processing of biomass, such as hydrothermal carbonization, pyrolysis, and gasification.118 Additionally, biochar is considered as a sustainable carbon material, which could be employed as a sorbent or a catalyst for environment and energy applications, including gas cleaning.119 To date, several works have been conducted for the NOx reduction with char.120–124 Most of them focused on the NO reduction over the char materials at higher temperatures. NO can react with carbon atoms in char to produce N2 and CO. A few work has been done for the NO oxidation by char-based materials. As mentioned above, Guo et al.25 used the commercialized AC prepared from coconut char (namely coconut-AC) for the NO oxidation. Moreover, the coconut-AC shows very high activity for the oxidation of NO to NO2 even with the low concentration of O2. In common, biochars possess many nitrogen or oxygen groups such as –NH2/–OH, C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, possibly contribute a lot on the adsorption performance and catalytic activity. Besides, inherent minerals in the char matrice show a certain specific catalytic effects.119 However, the property of char is often unstable, mainly depending on biomass types, processing methods, etc.
O, possibly contribute a lot on the adsorption performance and catalytic activity. Besides, inherent minerals in the char matrice show a certain specific catalytic effects.119 However, the property of char is often unstable, mainly depending on biomass types, processing methods, etc.
Chemical activation is one of the most effective ways to modify the characteristics of char. Chen et al.125 used KOH and ZnCl2 as activation agents to produce activated char, which can be used for De-NOx process. Sewage sludge were impregnated with the activation agent before the pyrolysis step. In this study, SO2, NO, N2O and HCl are main emissions from sewage sludge pyrolysis volatile combustion, in accordance with coals combustion. SO2 emission can be avoided by impregnation of KOH and ZnCl2. Activated char derived from the KOH-impregnated sewage sludge exhibited the best De-NOx performance. In the previous studies, the pitch-based ACFs showed higher De-SOx activity than other ACFs derived from different precursors. De-SOx activity can be further modified by the heat-treatment, continuous and complete removal of SOx. ACF is also effective for removing NOx in the presence of NH4.58 Furthermore, De-SOx and De-NOx could be achieved by using microwave irradiation over AC-based catalysts. It showed that adsorption capacities and removal efficiencies of Cu-based AC catalyst were higher than Mn-based or Zn-based AC catalyst.126 Nanoporous molecular basket sorbent was developed for NO2 and SO2 capture and separation from gas streams at room temperature based on polyethylene glycol (PEG)-loaded mesoporous molecular sieve SBA-15.127 Consequently, the integrated strategy of De-SOx and De-NOx could be achieved by porous carbon materials including activated char with the proper modification.
4. Concluding remarks
The catalytic oxidation of NO to NO2 at ambient temperatures has been a promising route for controlling NO emissions, since NO2 is subsequently removed as nitric acid in the presence of water. Because of their large specific surface area, high porosity, and relative chemical inertness, the carbon-based materials including ACs, ACFs are very attractive in De-NOx as catalysts or catalyst supports. However, the catalytic oxidation of NO to NO2 is still in its early stage of development and therefore there are many aspects that require additional research. The catalytic oxidation of NO to NO2 over these carbonaceous materials is mainly determined by the influence parameters of surface characteristics of carbon materials (e.g., pore structure, specific surface area, functional groups, and morphology), O2 concentration, and reaction temperature as described in Fig. 9. Additionally, in addition of specific metal actives, the catalytic performance could be significantly improved. Adsorption and catalytic reaction are two key points of NO oxidation over carbon-based materials. Furthermore, the strong dependence of NO conversion on the O2 concentration concludes that O2 is first adsorbed on the carbon surface, and then it reacts with NO to form the adsorbed NO2, desorbing to the gas phase thereafter.Consideration of the economic efficiency, carbon precursor derived from sustainable biomass can be fabricated into the desired carbon materials through functionalization. For example, biochar is considered as a sustainable carbon material that can be used as a sorbent or a catalyst support for gas cleaning. To date, only a few works have been conducted for the NOx reduction by char. In the future, more research works should be done for NO oxidation over char-based materials with and/or without activation. In addition, the integrated strategy of De-SOx and De-NOx could be developed by carbon materials with the proper modification methods.
Acknowledgements
This work is supported by the Startup Fund for Talents at NUIST under Grant No. 2243141501046. The authors are grateful to the financial supports by the National Science Foundation of China (21407079, 21577065, 91543115 and 91544220).References
- J. Zawadzki, M. Wisniewski and K. Skowronska, Heterogeneous reactions of NO2 and NO–O2 on the surface of carbons, Carbon, 2003, 41, 235–246 CrossRef CAS.
- M. Wojciechowska and S. Lomnicki, Nitrogen oxides removal by catalytic methods, Clean Products and Processes, 1999, 1, 237–242 Search PubMed.
- A. Fritz and V. Pitchon, The current state of research on automotive lean NOx catalysis, Appl. Catal., B, 1997, 13, 1–25 CrossRef CAS.
- N. Y. Topsoe, H. Topsoe and J. A. Dumesic, Vanadia/titania catalysts for selective catalytic reduction (SCR) of nitric-oxide by ammonia: I. Combined temperature-programmed in situ FTIR and on-line mass-spectroscopy studies, J. Catal., 1995, 151, 226–240 CrossRef CAS.
- B. Leckner, M. Karisson, K. Dam-Johansen, C. E. Weinell, P. Kilpinen and M. Hupa, Influence of additives on selective noncatalytic reduction of NO with NH3 in circulating fluidized bed boilers, Ind. Eng. Chem. Res., 1991, 30, 2396–2404 CrossRef CAS.
- H. Y. Huang and R. T. Yang, Removal of NO by reversible adsorption on Fe–Mn based transition metal oxides, Langmuir, 2001, 17, 4997–5003 CrossRef CAS.
- D. Ighigeanu, D. Martin, E. Zissulescu, R. Macarie, C. Oproiu, E. Cirstea, H. Iovu, I. Calinescu and N. Iacob, SO2 and NOx removal by electron beam and electrical discharge induced non-thermal plasmas, Vacuum, 2005, 77, 493–500 CrossRef CAS.
- J. Muñiz, G. Marbán and A. B. Fuertes, Low temperature selective catalytic reduction of NO over modified activated carbon fibres, Appl. Catal., B, 1999, 23, 25–35 CrossRef.
- M. F. Irfan, J. H. Goo and S. D. Kim, Co3O4 based catalysts for NO oxidation and NOx reduction in fast SCR process, Appl. Catal., B, 2008, 78, 267 CrossRef CAS.
- A. A. Verma, S. A. Bates, T. Anggara, C. Paolucci, A. A. Parekh, K. Kamasamudram, A. Yezerets, J. T. Miller, W. N. Delgass, W. F. Schneider and F. H. Ribeiro, NO oxidation: a probe reaction on Cu-SSZ-13, J. Catal., 2014, 312, 179–190 CrossRef CAS.
- I. Ellmers, R. P. Velez, U. Bentrup, A. Brückner and W. Grünert, Oxidation and selective reduction of NO over Fe-ZSM-5 – How related are these reactions?, J. Catal., 2014, 311, 199–211 CrossRef CAS.
- Y. Kong and C. Y. Cha, Reduction of NOx adsorbed on char with microwave energy, Carbon, 1996, 34, 1027–1033 CrossRef CAS.
- A. Claudino, J. L. Soares, R. F. P. M. Moreira and H. J. José, Adsorption equilibrium and breakthrough analysis for NO adsorption on activated carbons at low temperatures, Carbon, 2004, 42, 1483–1490 CrossRef CAS.
- J. Choi, K. S. Lee, D. Y. Choi, Y. J. Kim and S. S. Kim, Dry De-NOx process via gas-phase photochemical oxidation using an ultraviolet and aerosolized H2O/H2O2 hybrid system, Energy Fuels, 2014, 28, 5270–5276 CrossRef CAS.
- L. Chen, J. Lin and C. Yang, Absorption of NO2 in a packed tower with Na2SO3 aqueous solution, Environ. Prog., 2002, 21, 225–230 CrossRef CAS.
- G. A. Chappell, Aqueous scrubbing of nitrogen oxides from stack gases, in Pollution Control and Energy Needs-Advances in Chemistry, ed. R. Jimeson, et al., American Chemical Society, Washington DC, 1974 Search PubMed.
- G. Ruitang and X. Gao, Simultaneous removal of SO2 and NO2 by wet scrubbing using aqueous limestone slurry, Extended abstracts, 11th international conference on electrostatic precipitation, 2008, pp. 602–605 Search PubMed.
- J. P. S. Sousa, M. F. R. Pereira and J. L. Figueiredo, Catalytic oxidation of NO to NO2 on N-doped activated carbons, Catal. Today, 2011, 176, 383–387 CrossRef CAS.
- N. Tang, Y. Liu, H. Wang and Z. Wu, Mechanism study of NO catalytic oxidation over MnOx/TiO2 catalysts, J. Phys. Chem. C, 2011, 115, 8214–8220 CAS.
- L. Li, Q. Shen, J. Cheng and Z. Hao, Catalytic oxidation of NO over TiO2 supported platinum clusters I: preparation, characterization and catalytic properties, Appl. Catal., B, 2010, 93, 259–266 CrossRef CAS.
- J. Despres, M. Elsener, M. Koebel, O. Krocher, B. Schnyder and A. Wokaun, Catalytic oxidation of nitrogen monoxide over Pt/SiO2, Appl. Catal., B, 2004, 50, 73–82 CrossRef CAS.
- V. M. Zamansky, H. Loc, P. M. Maly and W. R. Seeker, Oxidation of NO to NO2 by hydrogen peroxide and its mixtures with methanol in natural gas and coal combustion gases, Combust. Sci. Technol., 1996, 120, 255–272 CrossRef CAS.
- B. Weidenhof, M. Reiser, K. Stowe, W. F. Maier, M. Kim and J. Azurdia, et al. High-throughput screening of nanoparticle catalysts made by flame spray pyrolysis as hydrocarbon/NO oxidation catalysts, J. Am. Chem. Soc., 2009, 131, 9207–9219 CrossRef CAS PubMed.
- H. Tsukahara, T. Ishida and M. Mayumi, Gas-Phase Oxidation of Nitric Oxide: Chemical Kinetics and Rate Constant, Nitric Oxide, 1999, 3, 191 CrossRef CAS PubMed.
- Z. Guo, Y. Xie, I. Hong and J. Kim, Catalytic oxidation of NO to NO2 on activated carbon, Energy Convers. Manage., 2001, 42, 2005–2018 CrossRef CAS.
- J. H. Pavlish, E. A. Sondreal, M. D. Mann, E. S. Olson, K. C. Galbreath, D. L. Laudal and S. A. Benson, State review of mercury control options for coal-fired power plants, Fuel Process. Technol., 2003, 82, 89–165 CrossRef CAS.
- X. Gao, S. Liu, Y. Zhang, X. Du, Z. Luo and K. Cen, Low temperature selective catalytic reduction of NO and NO2 with NH3 over activated carbon-supported vanadium oxide catalyst, Catal. Today, 2011, 175, 164–170 CrossRef CAS.
- Z. Wang, Y. Wang, D. Long, I. Mochida, W. Qiao, L. Zhan, X. Liu, S.-H. Yoon and L. Ling, Kinetics and mechanism study of low-temperature selective catalytic reduction of NO with urea supported on pitch-based spherical activated carbon, Ind. Eng. Chem. Res., 2011, 50, 6017–6027 CrossRef CAS.
- I. Mochida, Y. Kawabuchi, S. Kawano, Y. Matsumura and M. Yoshikawa, High catalytic activity of pitch-based activated carbon fibers of moderate surface area for oxidation of NO to NO2 at room temperature, Fuel, 1997, 76, 543–548 CrossRef CAS.
- I. Mochida, N. Shirahama, S. Kawano, Y. Korai, A. Yasutake, M. Tanoura, S. Fujii and M. Yoshikawa, NO oxidation over activated carbon fiber (ACF). Part 1. Extended kinetics over a pitch based ACF of very large surface area, Fuel, 2000, 79, 1713–1723 CrossRef CAS.
- I. Mochida, S. Kisamori, M. Hironaka, S. Kawano, Y. Matsumura and M. Yoshikawa, Oxidation of NO into NO2 over active carbon fibers, Energy Fuels, 1994, 8, 1341–1344 CrossRef CAS.
- S. Adapa, V. Gaur and N. Verma, Catalytic oxidation of NO by activated carbon fiber (ACF), Chem. Eng. J., 2006, 116, 25–37 CrossRef CAS.
- B. Galliker, R. Kissner, T. Nauser and W. H. Koppenol, Intermediates in the autoxidation of nitrogen monoxide, Chem.–Eur. J., 2009, 15, 6161–6168 CrossRef CAS PubMed.
- W. J. Zhang, S. Rabiei, A. Bagreev, M. S. Zhuang and F. Rasouli, Study of NO adsorption on activated carbons, Appl. Catal., B, 2008, 83, 63–71 CrossRef CAS.
- J. D. Atkinson, Z. Zhang and M. J. Rood, 105th Annual Meeting of the Air & Waste Management Association, San Antonio, 2012, Paper 2012-A-2058-AWMA Search PubMed.
- J. D. Atkinson, Z. Zhang, Z. Yan and M. J. Rood, Evolution and impact of acidic oxygen functional groups on activated carbon fiber cloth during NO oxidation, Carbon, 2013, 54, 444–453 CrossRef CAS.
- Z. Yan, Introduction to Catalysis, Press of Chemical Industry, Beijing, 2005 Search PubMed.
- J. A. Loiland and R. F. Lobo, Oxidation of zeolite acid sites in NO/O2 mixtures and the catalytic properties of the new site in NO oxidation, J. Catal., 2015, 325, 68–78 CrossRef CAS.
- S. N. Ahmed, R. Baldwin, F. Derbyshire, B. McEnaney and J. Stencel, Catalytic reduction of nitric oxide over activated carbons, Fuel, 1993, 72, 287–292 CrossRef CAS.
- P. Nikolov, M. Khristova and D. Mehandjiev, Low-temperature NO removal over copper-containing activated carbon, Colloids Surf., A, 2007, 295, 239–245 CrossRef CAS.
- A. Claudino, J. L. Soares, R. F. P. M. Moreira and H. J. Jose, Adsorption equilibrium and breakthrough analysis for NO adsorption on activated carbons at low temperatures, Carbon, 2004, 42, 1483–1490 CrossRef CAS.
- D. Lozano-Castelló, D. Cazorla-Amorós, A. Linares-Solano and D. F. Quinn, Influence of pore size distribution on methane storage at relatively low pressure: preparation of activated carbon with optimum pore size, Carbon, 2000, 40, 989–1002 CrossRef.
- E. Raymundo-Piñero, D. Cazorla-Amorós, C. Salinas-Martínez de Lecea and A. Linares-Solano, Factors controlling the SO2 removal by porous carbons: relevance of the SO2 oxidation step, Carbon, 2000, 38, 335–344 CrossRef.
- M. A. Lillo-Ródenas, D. Cazorla-Amorós and A. Linares-Solano, Behaviour of activated carbons with different pore size distributions and surface oxygen groups for benzene and toluene adsorption at low concentrations, Carbon, 2005, 43, 1758–1767 CrossRef.
- R. S. Rathore, D. K. Srivastava, A. K. Agarwal and N. Verma, Development of surface functionalized activated carbon fiber for control of NO and particulate matter, J. Hazard. Mater., 2010, 173, 211–222 CrossRef CAS PubMed.
- A. M. Rubel, M. L. Stewart and J. M. Stencel, Activated carbon for control of nitrogen oxide emissions, J. Mater. Res., 1995, 10, 562–567 CrossRef CAS.
- E. Richter, H.-J. Schmidt and H.-G. Schecker, Adsorption and catalytic reactions of NO and NH3 on activated carbon, Chem. Eng. Technol., 1990, 13, 332–340 CrossRef CAS.
- J. P. S. Sousa, M. F. R. Pereira and J. L. Figueiredo, NO oxidation over nitrogen doped carbon xerogels, Appl. Catal., B, 2012, 125, 398–408 CrossRef CAS.
- H. Teng, Y.-F. Hsu and Y.-T. Tu, Reduction of NO with NH3 over carbon catalysts — the influence of carbon surface structures and the global kinetics, Appl. Catal., B, 1999, 20, 145–154 CrossRef CAS.
- X. Wan, H. Shi, X. Zou, G. E. Peng and D. Wang, Effect of nitrogen doping on the reduction of nitric oxide with activated carbon in the presence of oxygen, J. Zhejiang Univ., Sci., A, 2008, 9, 113–117 CrossRef CAS.
- J. P. S. Sousa, M. F. R. Pereira and J. L. Figueiredo, Modified activated carbon as catalyst for NO oxidation, Fuel Process. Technol., 2013, 106, 727–733 CrossRef CAS.
- W.-J. Zhang, A. Bagreev and F. Rasouli, Reaction of NO2 with activated carbon at ambient temperature, Ind. Eng. Chem. Res., 2008, 47, 4358–4362 CrossRef CAS.
- S. Yang, J. Li, C. Wang, J. Chen, L. Ma, H. Chang, L. Chen, Y. Peng and N. Yan, Fe–Ti spinel for the selective catalytic reduction of NO with NH3: mechanism and structure–activity relationship, Appl. Catal., B, 2012, 117–118, 73–80 CrossRef CAS.
- J. Després, M. Elsener, M. Koebel, O. Kröcher, B. Schnyder and A. Wokaun, Catalytic oxidation of nitrogen monoxide over Pt/SiO2, Appl. Catal., B, 2004, 50, 73–82 CrossRef.
- Z. Zhang, J. D. Atkinson, B. Jiang, M. J. Rood and Z. Yan, Nitric oxide oxidation catalyzed by microporous activated carbon fiber cloth: an updated reaction mechanism, Appl. Catal., B, 2014, 148–149, 573–581 CrossRef CAS.
- J. Miyawaki, T. Shimohara, N. Shirahama, A. Yasutake, M. Yoshikawa, I. Mochida and S.-H. Yoon, Removal of NOx from air through cooperation of the TiO2 photocatalyst and urea on activated carbon fiber at room temperature, Appl. Catal., B, 2011, 110, 273–278 CrossRef CAS.
- W. Wang, G. McCool, N. Kapur, G. Yuan, B. Shan, M. Nguyen, U. M. Graham, B. H. Davis, G. Jacobs, K. Cho and X. Hao, Mixed-phase oxide catalyst based on Mn–mullite (Sm, Gd)Mn2O5 for NO oxidation in diesel exhaust, Science, 2012, 337, 832–835 CrossRef CAS PubMed.
- I. Mochida, Y. Korai, M. Shirahama, S. Kawano, T. Hada, Y. Seo, M. Yoshikawa and A. Yasutake, Removal of SOx and NOx over activated carbon fibers, Carbon, 2000, 38, 227–239 CrossRef CAS.
- J. Ding, Q. Zhong, S. Zhang, F. Song and Y. Bu, Simultaneous removal of NOx and SO2 from coal-fired flue gas by catalytic oxidation-removal process with H2O2, Chem. Eng. J., 2014, 243, 176–182 CrossRef CAS.
- W. Shen and W. Fan, Nitrogen-containing porous carbons: synthesis and application, J. Mater. Chem. A, 2013, 1, 999–1013 CAS.
- B. Cosane, C. Alba-Simionesco, F. Audonnet, G. Dosseh and K. E. Gubbins, Adsorption, structure and dynamics of benzene in ordered and disordered porous carbons, Phys. Chem. Chem. Phys., 2011, 13, 3748–3757 RSC.
- S. M. Manocha, Porous carbons, Sadhana: Academy Proceedings in Engineering Sciences, 2003, 28, 335 CAS.
- J. A. Loiland and R. F. Lobo, Low temperature catalytic NO oxidation over microporous materials, J. Catal., 2014, 311, 412–423 CrossRef CAS.
- N. Artioli, R. F. Lobo and E. Iglesia, Catalysis by confinement: enthalpic stabilization of NO oxidation transition states by microporous and mesoporous siliceous materials, J. Phys. Chem. C, 2013, 117, 20666–20674 CAS.
- R. Yan, T. Chin, Y. L. Ng, H. Q. Duan, D. T. Liang and J. H. Tay, Influence of surface properties on the mechanism of H2S removal by alkaline activated carbons, Environ. Sci. Technol., 2004, 38, 316–323 CrossRef CAS PubMed.
- T. J. Bandosz, On the adsorption/oxidation of hydrogen sulfide on activated carbons at ambient temperature, J. Colloid Interface Sci., 2002, 246, 1–20 CrossRef CAS PubMed.
- T. J. Bandosz, Effect of pore structure and surface chemistry of virgin activated carbons on removal of hydrogen sulfide, Carbon, 1999, 37, 483–491 CrossRef CAS.
- S. V. Mikhalovsky and Y. P. Zaitsev, Catalytic properties of activated carbons I. Gas-phase oxidation of hydrogen sulphide, Carbon, 1997, 35, 1367–1374 CrossRef CAS.
- F. Adib, A. Bagreev and T. J. Bandosz, Effect of surface characteristics of wood-based activated carbons on adsorption of hydrogen sulfide, J. Colloid Interface Sci., 1999, 214, 407–415 CrossRef CAS PubMed.
- F. Adib, A. Bagreev and T. J. Bandosz, Effect of pH and surface chemistry on the mechanism of H2S removal by activated carbons, J. Colloid Interface Sci., 1999, 216, 360–369 CrossRef CAS PubMed.
- A. Bagreev, F. Adib and T. J. Bandosz, pH of activated carbon surface as an indication of its suitability for H2S removal from moist air streams, Carbon, 2001, 39, 1897–1905 CrossRef CAS.
- T. Sun, Y. Shen and J. Jia, Gas cleaning and hydrogen sulfide removal for COREX coal gas by sorption enhanced catalytic oxidation over recyclable activated carbon desulfurizer, Environ. Sci. Technol., 2014, 48, 2263–2272 CAS.
- A. Bagreev, J. A. Menendez, I. Dukhno, Y. Tarasenko and T. J. Bandosz, Bituminous coal-based activated carbons modified with nitrogen as adsorbent of hydrogen sulfide, Carbon, 2004, 42, 469–476 CrossRef CAS.
- A. D. Budaeva and E. V. Zoltoev, Porous structure and sorption properties of nitrogen-containing activated carbon, Fuel, 2010, 89, 2623–2627 CrossRef CAS.
- H. F. Gorgulho, F. Gonçalves, M. F. R. Pereira and J. L. Figueiredo, Synthesis and characterization of nitrogen-doped carbon xerogels, Carbon, 2009, 47, 2032–2039 CrossRef CAS.
- K. Jurewicz, R. Pietrzak, P. Nowicki and H. Wachowska, Capacitance behaviour of brown coal based active carbon modified through chemical reaction with urea, Electrochim. Acta, 2008, 53, 5469–5475 CrossRef CAS.
- R. Pietrzak, XPS study and physico-chemical properties of nitrogen-enriched microporous activated carbon from high volatile bituminous coal, Fuel, 2009, 88, 1871–1877 CrossRef CAS.
- W. Shen, Z. Li and Y. Liu, Surface chemical functional groups modification of porous carbon, Recent Pat. Chem. Eng., 2008, 1, 27–40 CrossRef CAS.
- J. Alcañiz-Monge, A. Bueno-López, M. A. Lillo-Rodenas and M. J. Illán-Gómez, NO adsorption on activated carbon fibers from iron-containing pitch, Mircoporous and Mesoporous Materials, 2008, 108, 294–302 CrossRef.
- K. Li, L. Ling, C. Lu, W. Qiao, Z. Liu, L. Liu and I. Mochida, Catalytic removal of SO2 over ammonia-activated carbon fibers, Carbon, 2001, 39, 1803–1808 CrossRef CAS.
- H.-P. Boehm, Catalytic properties of nitrogen-containing carbons, in Carbon Materials for Catalysis, ed. P. Serp and J. L. Figueiredo, Wiley, Hoboken, New Jersey, 2009, pp. 219–265 Search PubMed.
- S. Matzner and H. P. Boehm, Influence of nitrogen doping on the adsorption and reduction of nitric oxide by activated carbons, Carbon, 1998, 36, 1697–1703 CrossRef CAS.
- J. L. Figueiredo, M. F. R. Pereira, M. M. A. Freitas and J. J. M. Órfão, Modification of the surface chemistry of activated carbons, Carbon, 1999, 37, 1379–1389 CrossRef CAS.
- B. Stöhr, H. P. Boehm and R. Schlögl, Enhancement of the catalytic activity of activated carbons in oxidation reactions by thermal treatment with ammonia or hydrogen cyanide and observation of a superoxide species as a possible intermediate, Carbon, 1991, 29, 707–720 CrossRef.
- B. J. Ku, J. K. Lee, D. Park and H.-K. Rhee, Treatment of activated carbon to enhance catalytic activity for reduction of nitric oxide with ammonia, Ind. Eng. Chem. Res., 1994, 33, 2868–2874 CrossRef CAS.
- R. S. Rathore, D. K. Srivastava, A. K. Agarwal and N. Verma, Development of surface functionalized activated carbon fiber for control of NO and particulate matter, J. Hazard. Mater., 2010, 173, 211–222 CrossRef CAS PubMed.
- S. Matzner and H. P. Boehm, Influence of nitrogen doping on the adsorption and reduction of nitric oxide by activated carbons, Carbon, 1998, 36, 1697–1709 CrossRef CAS.
- X. Gao, S. Liu, Y. Zhang, Z. Luo and K. Cen, Physicochemical properties of metal-loaded activated carbons and relationship with their performance in the removal of SO2 and NO, J. Hazard. Mater., 2011, 188, 58–66 CrossRef CAS PubMed.
- M. Wang, Z. Huang, T. Shimohara, F. Kang and K. Liang, NO removal by electrospun porous carbon nanofibers at room temperature, Chem. Eng. J., 2011, 170, 505–511 CrossRef CAS.
- M. Wang, Z. Huang, K. Shen, F. Kang and K. Liang, Catalytically oxidation of NO into NO2 at room temperature by graphitized porous nanofibers, Catal. Today, 2013, 201, 109–114 CrossRef CAS.
- A. Ramos, I. Cameán and A. B. García, Graphitization thermal treatment of carbon nanofibers, Carbon, 2013, 59, 2–32 CrossRef CAS.
- M. Endo, Y. A. Kim, T. Hayashi, T. Yanagisawa, H. Muramatsu and M. Ezaka, et al. Microstructural changes induced in “stacked cup” carbon nanofibers by heat treatment, Carbon, 2003, 41, 1941–1947 CrossRef CAS.
- S. Lim, S. H. Yoon, I. Mochida and J. H. Chi, Surface modification of carbon nanofiber with high degree of graphitization, J. Phys. Chem. B, 2004, 108, 1533–1536 CrossRef CAS.
- X. Wang, X. Li, L. Zhang, Y. Yoon, P. K. Weber and H. Wang, et al. N-doping of graphene through electrothermal reactions with ammonia, Science, 2009, 324, 768–771 CrossRef CAS PubMed.
- N. O. Weiss, H. Zhou, L. Liao, Y. Liu, S. Jiang and Y. Huang, et al. Graphene: an emerging electronic material, Adv. Mater., 2012, 24, 5782–5825 CrossRef CAS PubMed.
- S. Y. Pan and I. A. Aksay, Factors controlling the size of graphene oxide sheets produced via the graphite oxide route, ACS Nano, 2011, 5, 4073–4083 CrossRef CAS PubMed.
- D. Li and R. B. Kaner, Materials science. Graphene-based materials, Science, 2008, 320, 1170–1171 CrossRef CAS PubMed.
- W. S. Hummers and R. E. Offeman, Preparation of graphitic oxide, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- A. K. Geim and K. S. Novoselov, The rise of graphene, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk and J. R. Potts, et al. Graphene and graphene oxide: synthesis, properties, and applications, Adv. Mater., 2010, 22, 3906–3924 CrossRef CAS PubMed.
- Z. Guo, M. Wang, Z. Huang and F. Kang, Preparation of graphene/carbon hybrid nanofibers and their performance for NO oxidation, Carbon, 2015, 87, 282–291 CrossRef CAS.
- J. Zhou, Z. Sui, J. Zhu, P. Li, D. Chen and Y. Dai, et al. Characterization of surface oxygen complexes on carbon nanofibers by TPD, XPS and FT-IR, Carbon, 2007, 45, 785–796 CrossRef CAS.
- Y.-W. Ju, S.-H. Park, H.-R. Jung and W.-J. Lee, Electrospun activated carbon nanofibers electrodes based on polymer blends, J. Electrochem. Soc., 2009, 156, A489–A494 CrossRef CAS.
- M. Bikshapathi, G. N. Mathur, A. Sharma and N. Verma, Surfactant-enhanced multiscale carbon webs including nanofibers and Ni-nanoparticles for the removal of gaseous persistent organic pollutants, Ind. Eng. Chem. Res., 2012, 51, 2104 CrossRef CAS.
- A. Chakraborty, D. Deva, A. Sharma and N. Verma, Adsorbents based on carbon microfibers and carbon nanofibers for the removal of phenol and lead from water, J. Colloid Interface Sci., 2011, 359, 228 CrossRef CAS PubMed.
- S. Lim, S. H. Yoon, Y. Shimizu, H. Jung and I. Mochida, Surface control of activated carbon fiber by growth of carbon nanofiber, Langmuir, 2004, 20, 5559 CrossRef CAS PubMed.
- H. Katepalli, M. Bikshapathi, C. S. Sharma, N. Verma and A. Sharma, Synthesis of hierarchical fabrics by electrospinning of PAN nanofibers on activated carbon microfibers for environmental remediation applications, Chem. Eng. J., 2011, 171, 1194 CrossRef CAS.
- R. M. Singhal, A. Sharma and N. Verma, Micro-nano hierarchal web of activated carbon fibers for catalytic gas adsorption and reaction, Ind. Eng. Chem. Res., 2008, 47, 3700 CrossRef CAS.
- P. Talukdar, B. Bhaduri and N. Verma, Catalytic oxidation of NO over CNF/ACF-supported CeO2 and Cu nanoparticles at room temperature, Ind. Eng. Chem. Res., 2014, 53, 12537–12547 CrossRef CAS.
- R. W. Pekala, Organic aerogels from the polycondensation of resorcinol with formaldehyde, J. Mater. Sci., 1989, 24, 3221–3227 CrossRef CAS.
- P. V. Samant, F. Gonçalves, M. M. A. Freitas, M. F. R. Pereira and J. L. Figueiredo, Surface activation of a polymer based carbon, Carbon, 2004, 42, 1321–1325 CrossRef CAS.
- H. F. Gorgulho, F. Gonçalves, M. F. R. Pereira and J. L. Figueiredo, Synthesis and characterization of nitrogen-doped carbon xerogels, Carbon, 2009, 47, 2032–2039 CrossRef CAS.
- K. Jurewicz, R. Pietrzak, P. Nowicki and H. Wachowska, Capacitance behaviour of brown coal based active carbon modified through chemical reaction with urea, Electrochim. Acta, 2008, 53, 5469–5475 CrossRef CAS.
- M. Pérez-Cadenas, C. Moreno-Castilla, F. Carrasco-Marín and A. F. Pérez-Cadenas, Surface chemistry, porous texture, and morphology of N-doped carbon xerogels, Langmuir, 2008, 25, 466–470 CrossRef PubMed.
- J. P. S. Sousa, M. F. R. Pereira and J. L. Figueiredo, Carbon xerogel catalyst for NO oxidation, Catalysts, 2012, 2, 447–465 CrossRef CAS.
- E. Lam and J. H. T. Luong, Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals, ACS Catal., 2014, 4, 3393–3410 CrossRef CAS.
- Y. Shen, Carbothermal synthesis of metal-functionalized nanostructures for energy and environmental applications, J. Mater. Chem. A, 2015, 3, 13114–13188 CAS.
- H. S. Kambo and A. Dutta, A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications, Renewable Sustainable Energy Rev., 2015, 45, 359–378 CrossRef CAS.
- Y. Shen, Biochars as promising carbonaceous adsorbents or catalysts for tar elimination during biomass gasification, Renewable Sustainable Energy Rev., 2015, 43, 281–295 CrossRef CAS.
- L. Dong, S. Gao, W. Song and G. Xu, Experimental study of NO reduction over biomass char, Fuel Process. Technol., 2007, 88, 707–715 CrossRef CAS.
- M. Guerrero, A. Millera, M. U. Alzueta and R. Bilbao, Experimental and kinetic study at high temperatures of the NO reduction over Eucalyptus char produced at different heating rates, Energy Fuels, 2011, 25, 1024–1033 CrossRef CAS.
- W. Fan, Y. Lu and M. Xiao, Effect of peroxidation O2 concentration on the reduction reaction of NO by char at high temperature, Ind. Eng. Chem. Res., 2013, 52, 6101–6111 CrossRef CAS.
- X. Wu, Q. Song, H. Zhao, Z. Zhang and Q. Yao, Kinetic modeling of inherent mineral catalyzed NO reduction by biomass char, Environ. Sci. Technol., 2014, 48, 4184–4190 CrossRef CAS PubMed.
- X. Wang, J. Si, H. Tan, Q. Zhao and T. Xu, Kinetics investigation on the reduction of NO using straw char based on physicochemical characterization, Bioresour. Technol., 2011, 102, 7401–7406 CrossRef CAS PubMed.
- H. Chen, D. Chen and L. Hong, Influences of activation agent impregnated sewage sludge pyrolysis on emission characteristics of volatile combustion and De-NOx performance of activated char, Appl. Energy, 2015, 156, 767–775 CrossRef CAS.
- S. C. Ma, J. Yao, X. Ma, L. Cao and M. Guo, Removal of SO2 and NOx using microwave swing adsorption over activated carbon carried catalyst, Chem. Eng. Technol., 2013, 36, 1217–1224 CrossRef CAS.
- X. Wang, X. Ma, S. Zhao, B. Wang and C. Song, Nanoporous molecular basket sorbent for NO2 and SO2 capture based on a polyethylene glycol-loaded mesoporous molecular sieve, Energy Environ. Sci., 2009, 2, 878–882 CAS.
| This journal is © The Royal Society of Chemistry 2016 |