Synthesis of porous MnCo2O4 microspheres with yolk–shell structure induced by concentration gradient and the effect on their performance in electrochemical energy storage†
Abstract
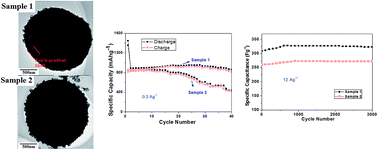
In this study, novel spherical yolk–shell MnCo2O4 powders with concentration gradient have been synthesized. The porous microspheres with yolk–shell structure (2.00–3.00 μm in average diameter, ∼200 nm in thickness of shell) are built up by irregular nanoparticles attached to each other. It is shown that the formation of yolk–shell structure may be induced by the core–shell concentration gradient. And the Co : Mn atomic ratios of core and shell are about 1.65 : 1 and 2.61 : 1, respectively. Interestingly, a similar uniform spherical MnCo2O4 without yolk–shell structure and concentration gradient prepared as a contrast, the superior electrochemical performance of the former by using in Li-ion batteries and supercapacitors has been proved including higher initial discharge capacity (1445.1 mA h g−1 at 0.2 A g−1) and initial specific capacitance (761.3 F g−1 at 2 A g−1), and more advanced capacity retention (∼860.0 mA h g−1 after 40 cycles at 0.2 A g−1, and ∼330.0 F g−1 after 3000 cycles at 12 A g−1).


 Please wait while we load your content...
Please wait while we load your content...