Mild and ambient annulations for pyrrole synthesis from amines and arylacetaldehydes†
Abstract
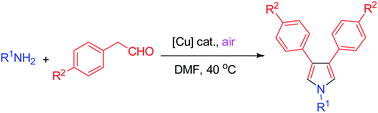
A mild and ambient pyrrole synthesis via copper-catalyzed aerobic cyclization of alkylamines and arylacetaldehydes is described. This method provided alternative access to a range of 1,3,4-trisubstituted pyrroles under environmentally friendly reaction conditions.

 Please wait while we load your content...
Please wait while we load your content...