Photothermal and photodynamic therapy reagents based on rGO–C6H4–COOH†
Abstract
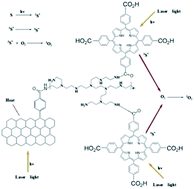
A photothermal therapy (PTT) and photodynamic therapy (PDT) reagent was synthesized based on rGO–C6H4–COOH (graphene diazotized). rGO–C6H4–COOH was first conjugated with polyethyleneimine (PEI) to enhance water solubility and then tetrakis(4-carboxyphenyl) porphyrin (TCPP) was further attached to obtain PDT ability for the system. The rGO–PEI–TCPP system exhibited excellent stability in various biological solutions and the cytotoxicity of this rGO–PEI–TCPP system was acceptable. The CBRH7919 cancer cells could be induced to effectively undergo apoptosis by rGO–PEI–TCPP under laser irradiation. The reason is mainly attributed to the excellent photothermal and photodynamic effects, i.e., the production of heat and singlet oxygen. At the same time, the CBRH7919 cancer cells could be pushed to the vulnerable G2 phase of the cell cycle, which is the most sensitive and susceptible stage to damage by radiation.

 Please wait while we load your content...
Please wait while we load your content...