Binder-free integration of insoluble cubic CuCl nanoparticles with a homologous Cu substrate for lithium ion batteries
Abstract
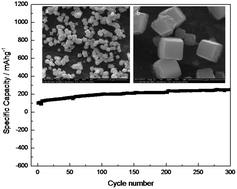
Binder-free integration of a novel insoluble cubic cuprous chloride (CuCl) nanoparticle anode material with homologous Cu foil was designed and achieved via facile in situ electrochemical self-assembly for the first time. The integrated CuCl/Cu electrode for lithium ion batteries was studied in terms of SEM, EDX, XRD, galvanostatic charge/discharge, cycle stability, rate performance, cyclic voltammograms (CV) and AC impedance. As expected, insoluble cubic CuCl nanoparticles did in situ grow and tightly combine with the surface of Cu foil, and the resultant CuCl/Cu electrode delivered a reversible discharge capacity of 250.6 mA h g−1 after 300 cycles at 2C, indicating satisfactory cyclic stability. In addition, the corresponding Li+ diffusion coefficient was calculated to be 1.8 × 10−11 cm2 s−1, higher than that of the MnO anode material in literature. Binder-free integration of homologous materials via self-assembly can not only ensure the tight combination of insoluble CuCl nanoparticles with Cu foil, but also avoid negative effects due to the polymer binder on electrochemical performance.

 Please wait while we load your content...
Please wait while we load your content...