Unique solubility of polyoxoniobate salts in methanol: coordination to cations and POM methylation†
P. A. Abramov*ab,
C. Vicentc,
N. B. Kompankova,
J. A. Larichevaa and
M. N. Sokolovab
aNikolaev Institute of Inorganic Chemistry SB RAS, Novosibirsk, 630090, Russia. E-mail: abramov@niic.nsc.ru
bNovosibirsk State University, Novosibirsk, Russia 630090
cServeis Centrals d’Instrumentació Científica, Universitat Jaume I, Av. Sos Baynat s/n, 12071 Castelló, Spain
First published on 28th January 2016
Abstract
Sodium salts of hybrid organometallic POM complexes consisting of [M6O19]8− (M = Nb, Ta) and half-sandwich fragments {Cp*M}2+ (Cp* = η5-C5(CH3)5; M = Rh, Ir) and {(C6H6)Ru}2+ dissolve only in one organic solvent, that is, in methanol. From methanolic solutions, crystals of Na4[trans-{(C6H6)Ru}2Nb6O19]·14.125MeOH·2H2O (1), K4[trans-{Cp*Rh}2Nb6O19]·4MeOH·10H2O (2) and K4[trans-{Cp*Ir}2Nb6O19]·10MeOH·4H2O (3) were isolated and characterized by X-ray analysis. Methoxo species [{LM′}2M6O19−n(OCH3)n] (n = 1–3) were detected in solution by ESI-MS and NMR, and they account only for about half of the total speciation in the solution. DFT calculations were used to calculate 13C NMR chemical shifts in the methoxo complexes and to assess their relative stability. Reasons for the preferred solubility in methanol are discussed.
Introduction
The chemistry of polyoxoniobates and tantalates is routinely confined to strongly alkaline aqueous solutions, but a remarkably rich chemistry is displayed even under these restrictions.1 Non-aqueous chemistry of Nb and Ta polyoxometalates is by far less studied. Hydrated Nb2O5 can be solubilized in methanol under solventothermal conditions in the presence of Me4NOH as a base.2 It also reacts with different organic bases in acetonitrile. In this way [Nb10O28]6−,3 [Nb20O54]8−,4 as well as the recently reported [Ta10O28]6−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 were prepared. The main reason of the strong bias in favor of choosing water as the solvent is the high charge of the polyniobates, which requires large numbers of counter cations, typically alkali metal cations. This leads to high lattice energies, which can be counterbalanced only by a solvent with a high dielectric constant and donor/acceptor number, preferably with water. Hydrogen bonds also play an important part in stabilizing the highly charged polyanions, and those are again best provided by water. Can hydrated alkali metal salts of highly charged polyoxometalates be dissolved and handled in organic solvents? One can expect that coordination of a positively charged organometallic moiety such as {Cp*M}2+ (M = Rh, Ir) or {(arene)Ru}2+ (arene is benzene and its derivatives) to [Nb6O19]8− would reduce the overall negative charge and simultaneously increase the hydrophobicity of the hybrid complex, thus enabling solubility in organic solvents. Indeed, Proust et al. reported that the neutral complex [{(p-cym)Ru}4Nb6O19] (p-cym = 1-methyl-4-(1-methylethyl)benzene) was soluble in CH3OH, and the negative ion mode electrospray mass spectrum of methanolic solutions detected formation of the methoxo complexes [{(p-cym)Ru}2Nb6O16(OCH3)3]− and [{(p-cym)Ru}3Nb6O18(OCH3)]−, arising from de-complexation of one or two {(p-cym)Ru}2+ fragments and formation of Nb–OCH3 bonds.6 This solvolitic behavior in methanol is strongly reminiscent of the reactivity of [Nb2W4O19]4− towards CH3OH, and of [P2W15V3O62]9− with CH3C(CH2OH)3.7,8 In the present study we found that sodium salts of [{(C6H6)Ru}2Nb6O19]4−, [{Cp*Rh}2Nb6O19]4− and [{Cp*Ir}2Nb6O19]4− were soluble in methanol, but not in ethanol or other organic solvents. Both the solvolysis of the anion and solvation of the alkali metal cation are the driving force in our case: the methoxo species [{LM′}2M6O19−n(OCH3)n] (n = 1–3) are detected by ESI-MS and NMR, and they account for about half of the total speciation of the hybrid complexes in methanol. From these solutions, crystals of Na4[{(C6H6)Ru}2Nb6O19]·14.125MeOH·2H2O (1), K4[trans-{Cp*Rh}2Nb6O19]·4MeOH·10H2O (2) and K4[trans-{Cp*Ir}2Nb6O19]·10MeOH·4H2O (3) were isolated in high yields, which bear no Nb–OCH3 groups, but show direct coordination of CH3OH to Na+ instead. These observations are presented and discussed in detail below.
5 were prepared. The main reason of the strong bias in favor of choosing water as the solvent is the high charge of the polyniobates, which requires large numbers of counter cations, typically alkali metal cations. This leads to high lattice energies, which can be counterbalanced only by a solvent with a high dielectric constant and donor/acceptor number, preferably with water. Hydrogen bonds also play an important part in stabilizing the highly charged polyanions, and those are again best provided by water. Can hydrated alkali metal salts of highly charged polyoxometalates be dissolved and handled in organic solvents? One can expect that coordination of a positively charged organometallic moiety such as {Cp*M}2+ (M = Rh, Ir) or {(arene)Ru}2+ (arene is benzene and its derivatives) to [Nb6O19]8− would reduce the overall negative charge and simultaneously increase the hydrophobicity of the hybrid complex, thus enabling solubility in organic solvents. Indeed, Proust et al. reported that the neutral complex [{(p-cym)Ru}4Nb6O19] (p-cym = 1-methyl-4-(1-methylethyl)benzene) was soluble in CH3OH, and the negative ion mode electrospray mass spectrum of methanolic solutions detected formation of the methoxo complexes [{(p-cym)Ru}2Nb6O16(OCH3)3]− and [{(p-cym)Ru}3Nb6O18(OCH3)]−, arising from de-complexation of one or two {(p-cym)Ru}2+ fragments and formation of Nb–OCH3 bonds.6 This solvolitic behavior in methanol is strongly reminiscent of the reactivity of [Nb2W4O19]4− towards CH3OH, and of [P2W15V3O62]9− with CH3C(CH2OH)3.7,8 In the present study we found that sodium salts of [{(C6H6)Ru}2Nb6O19]4−, [{Cp*Rh}2Nb6O19]4− and [{Cp*Ir}2Nb6O19]4− were soluble in methanol, but not in ethanol or other organic solvents. Both the solvolysis of the anion and solvation of the alkali metal cation are the driving force in our case: the methoxo species [{LM′}2M6O19−n(OCH3)n] (n = 1–3) are detected by ESI-MS and NMR, and they account for about half of the total speciation of the hybrid complexes in methanol. From these solutions, crystals of Na4[{(C6H6)Ru}2Nb6O19]·14.125MeOH·2H2O (1), K4[trans-{Cp*Rh}2Nb6O19]·4MeOH·10H2O (2) and K4[trans-{Cp*Ir}2Nb6O19]·10MeOH·4H2O (3) were isolated in high yields, which bear no Nb–OCH3 groups, but show direct coordination of CH3OH to Na+ instead. These observations are presented and discussed in detail below.
Results and discussion
ESI-MS experiments
The first indications of the formation of methoxo complexes were obtained from ESI-MS experiments. Reactions of [M6O19]8− (M = Nb and Ta) salts with [Cp*RhCl2]2 at a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Rh/M6 ratio in water yielded solids containing [(Cp*Rh)2M6O19]4− anions.
1 Rh/M6 ratio in water yielded solids containing [(Cp*Rh)2M6O19]4− anions.
When their ESI (−) mass spectra were investigated using methanol as the solvent, signals attributed to methoxo species were readily identified on the basis of their m/z value as well as their isotopic distribution. In particular signals from the 2:1 species [{Cp*Rh}2Nb6O19H3]− (m/z 1340), [{Cp*Rh}2Nb6O18(OCH3)H2]− (m/z 1354), [{Cp*Rh}2Ta6O19H3]− (m/z 1868) and [{Cp*Rh}2Ta6O18(OCH3)H2]− (m/z 1882) were identified. Table S1† shows the m/z values for the methoxo species. Signals from the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adducts, namely [{Cp*Rh}Nb6O19H5]− (m/z 1104), [{Cp*Rh}Nb6O17(OCH3)2H3]− (m/z 1132), [{Cp*Rh}Nb6O18(OCH3)H4]− (m/z 1118), [{Cp*Rh}Ta6O18(OCH3)H3]2− (m/z 824), and [{Cp*Rh}Ta6O18(OCH3)H4]− (m/z 1646),9 were also observed in their respective ESI mass spectra in methanol. We also found that sodium salts of the 2
1 adducts, namely [{Cp*Rh}Nb6O19H5]− (m/z 1104), [{Cp*Rh}Nb6O17(OCH3)2H3]− (m/z 1132), [{Cp*Rh}Nb6O18(OCH3)H4]− (m/z 1118), [{Cp*Rh}Ta6O18(OCH3)H3]2− (m/z 824), and [{Cp*Rh}Ta6O18(OCH3)H4]− (m/z 1646),9 were also observed in their respective ESI mass spectra in methanol. We also found that sodium salts of the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes Na4[{Cp*Ir}2Nb6O19]·22H2O and Na6[{Cp*Ir}Ta6O19]·27H2O were soluble in CH3OH, and signals from the corresponding anions as well as from their mono- and di-methylated derivatives [{Cp*Ir}2Nb6O18(OCH3)H]2− (m/z 765) and [{Cp*Ir}2Nb6O17(OCH3)H2]− (m/z 1532) were detected (see for example Fig. S1 and S2†) in these solutions.10 Methoxo complexes were also detected in the ESI-MS spectra of [{Cp*Ir}2TeNb5O19]3− and [{Cp*Ir}TeNb5O19]5−.11 This indicates the tendency of the Lindqvist POMs to form methoxo species upon dissolving in methanol.
1 complexes Na4[{Cp*Ir}2Nb6O19]·22H2O and Na6[{Cp*Ir}Ta6O19]·27H2O were soluble in CH3OH, and signals from the corresponding anions as well as from their mono- and di-methylated derivatives [{Cp*Ir}2Nb6O18(OCH3)H]2− (m/z 765) and [{Cp*Ir}2Nb6O17(OCH3)H2]− (m/z 1532) were detected (see for example Fig. S1 and S2†) in these solutions.10 Methoxo complexes were also detected in the ESI-MS spectra of [{Cp*Ir}2TeNb5O19]3− and [{Cp*Ir}TeNb5O19]5−.11 This indicates the tendency of the Lindqvist POMs to form methoxo species upon dissolving in methanol.
Since in all cases formation of methylated hybrid anions was detected by ESI-MS experiments in freshly prepared methanolic solutions, it would appear that they rapidly form in solution in significant amounts, up to a certain equilibrium point between the starting oxo and methoxo species. However, we are aware that the large methanol excess employed for sample preparation for ESI-MS analysis (sample concentration ca. 5 × 10−5 M in CH3OH) might exaggerate the propensity of the oxo or hydroxo groups to be replaced by methoxo; in addition, the appearance of the signals due to the methoxo species may be due to secondary processes operative only in the gas phase under experimental conditions. Therefore we tried to isolate methoxo complexes directly from methanolic solutions and demonstrate their formation in solution by X-ray crystallography and NMR techniques, respectively.
X-ray structures
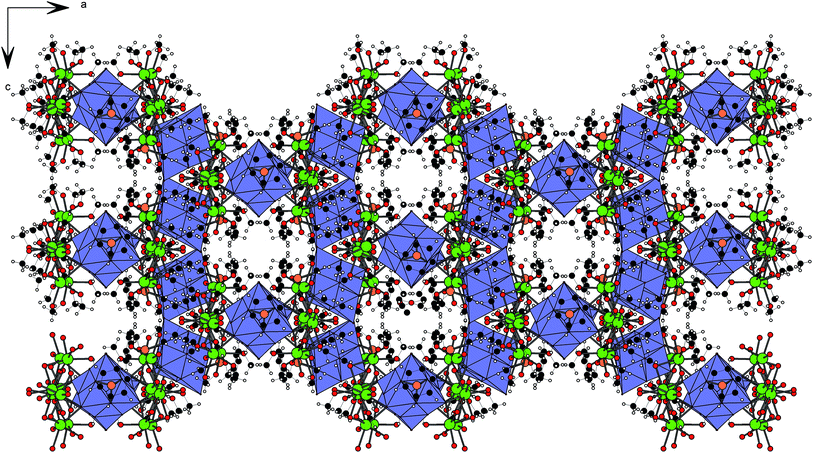
The reaction of sodium hexaniobate with [(C6H6)RuCl2]2 proceeds slowly upon dissolution of the ruthenium complex in a water solution of hexaniobate, which typically takes 1–2 hours for completion depending on the loads and temperature, and then the yellow solution is heated and stirred for 12 h. The product is precipitated with acetone (slow diffusion gives yellow crystals and quick addition gives a yellow precipitate). Recrystallization from CH3OH yields large yellow plates of Na4[{(C6H6)Ru}2Nb6O19]·14.125MeOH·2H2O (1), whose structure was determined. The phase purity of the product was confirmed by XRPD.The crystal structure of 1 consists of trans-[{(C6H6)Ru}2Nb6O19]4− hybrid anions and sodium cations, which aggregate into [(MeOH)3Na(μ-MeOH)Na(H2O) (MeOH)2]2+ dimers; hence the formula of 1 is better rendered as [(MeOH)3Na(μ-MeOH)Na(H2O)(MeOH)2]2[{(C6H6)Ru}2Nb6O19]·2.125MeOH. The cationic dimers unite the anions into a 3D framework (Fig. 1). This is the first example of breaking the standard layered structural motif common to all previously known hybrid complexes bearing two coordinated C6H6 or Cp* fragments.6,9 The formation of the 3D framework (the building block is represented in Fig. S3†) creates infinite channels running along the [010] and [100] crystallographic directions, which are filled with disordered methanol molecules (2.125 molecules per formula unit from XRD refinement) (Fig. S4†). The methanol molecules that are coordinated to the cations form strong hydrogen bonds with bridging oxo ligands (d(MeOH⋯μ2-O) is 2.645 and 2.701 Å) of the hybrid POM. Moreover, one solvate water molecule forms a weaker hydrogen bond with μ2-O (the H2O⋯O distance is 2.821 Å).
trans-K4[{Cp*Rh}2Nb6O19]·20H2O (ref. 9) and trans-K4[{Cp*Ir}2Nb6O19]·22H2O (ref. 10) also dissolve in methanol, but crystalline samples could not be obtained by straightforward evaporation of the solvent. In one of the crystallization attempts we added a few milligrams of dibenzo-18-crown-6 to the methanolic solutions, which then were left at 2 °C. Yellow single crystal plates were obtained in both cases, but curiously enough, no crown ether molecules entered the structure, the formulae of the products being trans-K4[{Cp*Rh}2Nb6O19]·4MeOH·10H2O (2) and trans-K4[trans-{Cp*Ir}2Nb6O19]·10MeOH·4H2O (3). Possibly the crown ether binds excess water, forming associates with strong hydrogen bonds, which remain in solution, but this hydration shifts the equilibrium and facilitates formation of 2 and 3. The two salts are obviously not isostructural, and 2 crystallizes in the P21/c space group while 3 adopts the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) group. This difference is due to the different values in the content of methanol and water molecules. However, whatever the differences are, in both structures the hybrid anions trans-[{Cp*M}2Nb6O19]4− are combined with the cationic part (there are infinite chains of the cations connected with the solvate methanol, water molecules and oxygen ligands of the anions, Fig. S5 and S6†) into the layers spreading along the [011] (M = Rh) or [110] (M = Ir) crystallographic directions (Fig. S7 and S8†). Both in 2 and 3 additional K+ cations coordinate free {Nb3O3} faces of the anions. In 2 such cations have CN 7 (with three oxo ligands from the anion face, three water molecules and one methanol, Fig. S9†), and in 3 K+ has CN 8 (three oxo ligands from the anion face, three water molecules and two methanol molecules, Fig. S10,† left). Methanol molecules, coordinated to the cations, form hydrogen bonds with POM both in 2 and 3. In 2 only the methanol molecules that are located in the positions near to the anion produce hydrogen bonds (d(Nb
group. This difference is due to the different values in the content of methanol and water molecules. However, whatever the differences are, in both structures the hybrid anions trans-[{Cp*M}2Nb6O19]4− are combined with the cationic part (there are infinite chains of the cations connected with the solvate methanol, water molecules and oxygen ligands of the anions, Fig. S5 and S6†) into the layers spreading along the [011] (M = Rh) or [110] (M = Ir) crystallographic directions (Fig. S7 and S8†). Both in 2 and 3 additional K+ cations coordinate free {Nb3O3} faces of the anions. In 2 such cations have CN 7 (with three oxo ligands from the anion face, three water molecules and one methanol, Fig. S9†), and in 3 K+ has CN 8 (three oxo ligands from the anion face, three water molecules and two methanol molecules, Fig. S10,† left). Methanol molecules, coordinated to the cations, form hydrogen bonds with POM both in 2 and 3. In 2 only the methanol molecules that are located in the positions near to the anion produce hydrogen bonds (d(Nb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯HOCH3) is 2.761 Å) stronger than those in water molecules (d(Nb
O⋯HOCH3) is 2.761 Å) stronger than those in water molecules (d(Nb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H2O) is 2.900 Å) (Fig. S9†). This effect is more significant in the case of 3, which has more CH3OH molecules in the structure. All methanol molecules are oriented in a way to afford hydrogen bonding with the anion (d(O⋯O) is 2.717–2.748 Å) (Fig. S10,† right). Details of XRD experiments and refinement are summarized in Table S2.†
O⋯H2O) is 2.900 Å) (Fig. S9†). This effect is more significant in the case of 3, which has more CH3OH molecules in the structure. All methanol molecules are oriented in a way to afford hydrogen bonding with the anion (d(O⋯O) is 2.717–2.748 Å) (Fig. S10,† right). Details of XRD experiments and refinement are summarized in Table S2.†
Qualitative experiments have shown that also Cs+ (Ta), K+ (Nb), and Na+ (Ta, Nb) salts for [{Cp*M}2M6O19]4− complexes (M = Rh, Ir), and K+ (Nb), and Na+ (Nb, Ta) salts for [{(C6H6)Ru}2M6O19]6− complexes are soluble in methanol. Attempts to get crystals from these solutions failed. The crystal packing of all starting materials consists of the layers formed by the hybrid anions and solvated cations which stack together only through π–π interactions between the aromatic rings of the organic ligands. Methanol can penetrate between such layers and replace water in the coordination spheres of the cations, while CH3CN or CH2Cl2 cannot do this.
13C and 1H NMR experiments
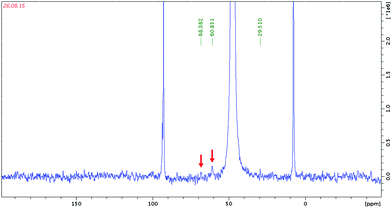
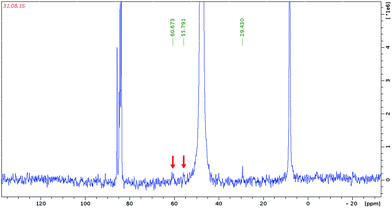
The compounds 1–3 were isolated in high yields. This means either that the methoxo complexes are present only in solution in a rapidly shifting equilibrium so that they dissociate upon crystallization, or that they are kinetically stable, but only as a minor component of the complicated equilibria. In order to check this hypothesis we studied methanolic solutions of 2 and 3 by NMR. In the 13C NMR spectra of 2 and 3 in CD3OD there appears two signals at 68 and 61 ppm for the Rh complex (Fig. 2), and at 61 and 56 ppm for the Ir complex (Fig. 3), in addition to the signals from the Cp* ligands.Since free methanol gives a 13C NMR resonance at 49.5 ppm, these signals can be assigned to coordinated CH3O−. Indeed, Errington et al. reported (Bu4N)2[(MeO)NbW5O18] in CD3CN δCH3O at 65.91 ppm.12 Similar chemical shifts for various methoxy species were reported elsewhere.13 Karaliota et al. even discriminated between terminal Nb–OCH3 and bridging Nb–(μ-OCH3)–Nb modes, assuming δ 67 ppm for the bridging and 60–64 ppm for the terminal methoxy ligands for solutions of NbCl5 in methanol.14 Thus 13C NMR gives evidence for the formation of methoxo complexes in methanolic solutions of 2 and 3. In order to confirm this assignment we carried out DFT calculation of the 13C chemical shifts taking methylated isomers of [{Cp*Ir}2Nb6O18(OCH3)]3− as models, which yielded δCH3 60.6 ppm and 63.4 ppm for the terminal and bridging methoxide, respectively. These results show that the difference between the chemical shifts of the terminal and bridged methoxo ligands is meaningful and agrees with Karaliota’s assignment. According to 13C NMR, we have complexes with both terminal (upfield) and bridging (downfield) CH3O− ligands in solution. Three isomers are possible, as shown in Fig. 4. The formation of the new species in CH3OH solutions can be also deduced from new signals from Cp* which appear at 93.70 and 94.23 ppm as broad resonances, together with signals from the starting trans-[{Cp*Rh}2Nb6O19]4− at 92.91 and 92.68 ppm.
The methyl group proton (1H NMR) resonances which appear in solutions of NbCl5 in CH3OH fall in three series around the 4.5 ppm, 4.2–3.8 ppm, and 3.3 ppm regions, and are due to bridging methoxides, terminal methoxides, and to free methanol, respectively.14,15 1H NMR of 1 in MeOH gives δ = 3.530, 3.520, 3.504, 3.491, and 3.468 ppm for terminal coordination and δ = 4.284 ppm for bridging coordination, but in 13C NMR we observed only one signal at 61 ppm. For complex 2 1H NMR also shows formation of terminal MeO− species (δ = 3.67 ppm), but peaks from bridging ligands also appear at 4.53–4.55 ppm. The Cp* signals from trans-[{Cp*Rh}2Nb6O19]4− appear at 1.90 and 1.87 ppm, together with new signals at 1.92 and 1.94 ppm. The latter two signals can be assigned to the signals from Cp* groups in the CH3O− complexes. The signal intensity at 3.67 ppm is 5% of that of the two signals with δ = 1.90 and 1.87 ppm, hence the corresponding Cp* signal must have a 10-fold intensity, which in turn corresponds to that of the peak with δ = 1.92 ppm. In the same way, the integral intensity of the signals from the bridging CH3O group (from various isomers at 4.53–4.55 ppm) is only 1%, which correlates with the intensity of the Cp* peak at 1.94 ppm (10%). The total intensity of the Cp* signals from the methoxo complexes is 60% of that of trans-[{Cp*Rh}2Nb6O19]4− which indicates a high degree of methylation (37.5%), in qualitative agreement with the ESI data. A methanolic solution of 3 demonstrates signals from terminal (3.68 ppm) and bridging (4.35, 4.33, 4.32, 4.30, 4.29, and 4.26 ppm) groups. The signals from Cp* appear at 1.83 and 1.81 ppm (for trans-[{Cp*Ir}2Nb6O19]4−) and 1.85–1.87 ppm (for methylated POMs). The signal at 1.85 ppm can be assigned to the isomers with a terminal CH3O group (10% of the intensity of the Cp* groups from the starting trans-[{Cp*Ir}2Nb6O19]4−), and that of 1.87 ppm to the isomers with a bridging methoxide (100% of the intensity). This corresponds to the total methylation of ca. 53% of the starting complex. The formation of the new species in CH3OH solutions can be also deduced from new signals from Cp* which appear at 84.75 and 85.65 ppm as broad resonances, together with the signals from the starting trans-[{Cp*Ir}2Nb6O19]4− at 83.67 and 83.64 ppm. Observed signals in 1H NMR for all complexes are summarized in Table 1. Also both for Rh and Ir there is a signal at δ = 29.4 ppm (1H NMR δ = 3.37 ppm) which appears also in ref. 14, and was assigned by the authors of this work to CH3Cl. But we assign it as an acetone trace (30 ppm, http://nmrshiftdb.nmr.uni-koeln.de/).
The conclusion from this part of the work is that the formation of the methoxo complexes does take place in methanolic solutions and contributes to enhanced solubility of Na4[{(C6H6)Ru}2Nb6O19] and K4[{Cp*M}2Nb6O19] (M = Rh, Ir) in methanol as a result of specific solvolysis.
DFT calculations
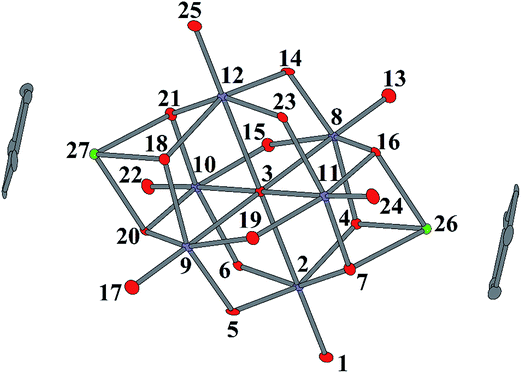
In order to gain more insight into the formation of the methoxo complexes, DFT calculations were carried out (atomic numbering is presented in Fig. 4). Calculation of the charge distribution on the terminal oxoligands of the free Lindqvist anion [Nb6O19]8− shows equal Hirshfeld charges of −0.754 (see Table S3†). Coordination of a {Cp*Ir}2+ fragment leads, as expected, to an overall decrease in the negative charge for all terminal oxygen atoms, but the terminal oxo ligands at the free face opposite to {Cp*IrNb3O3} retain a greater negative charge (−0.620 vs. −0.565) (Table S3†).This means that the coordination of {Cp*Ir}2+ “polarizes” the Lindqvist structure in such a way that more negatively charged oxygen atoms belong to the face opposite to the {Cp*IrNb3O3} group. This perhaps explains preferential formation of the trans-isomers observed for the bicapped [{XM}2M6O19]4− species.9–11
Capping of [Nb6O19]8− with half-sandwich moieties also significantly increases the positive charge at the Nb atoms (by almost 0.2 e). This may lead to stabilization of an intermediate with CN 7, where a CH3OH molecule is coordinated to Nb; in fact there are examples of POMs with seven-coordinated Nb.16 Following this, we can envision proton transfer to one of the terminal or bridging oxides, followed by substitution of CH3O− for OH−. We have calculated total energies and Hirshfeld charges for various isomers of monomethylated trans-[{Cp*Ir}2Nb6O18(OCH3)]3−. The formation of a single possible terminal methoxo complex by methylation of one of the six equivalent terminal Nb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups (Fig. 4, isomer I) is slightly favored over the bridging isomer where methylation occurs at either of the six equivalent Nb–O–Nb groups (isomer II). The isomer with capping μ3-OCH3 (isomer III) is the least favored (Table S3†).
O groups (Fig. 4, isomer I) is slightly favored over the bridging isomer where methylation occurs at either of the six equivalent Nb–O–Nb groups (isomer II). The isomer with capping μ3-OCH3 (isomer III) is the least favored (Table S3†).
This is in agreement with Errington’s isolation of (Bu4N)2[(MeO)NbW5O18] with a terminal Nb–OCH3 group,12 but does not exclude the presence of methoxo-bridged isomers in equilibrium, as can be seen from the NMR spectra in our case.
To conclude, exclusive solubility of the hybrid organometallic–POM complexe salts with alkali metal cations in methanol is due to the formation of the methoxo complexes (as detected in solution) and solvation of Na+ and K+ (as is seen in the crystals of the solids which were isolated from such solutions), and hydrophobic interactions with Cp* and C6H6 moieties. This, prima facie, does not explain the lack of solubility in ethanol, which is a better donor (being more basic). But CH3OH strongly differs from C2H5OH in having a significantly larger value of the dielectric constant (ε), of 31 vs. 24.
Thus methanol is better suited for dismantling the crystal lattice of these ionic compounds due to a sufficiently high ε value. The packing in the crystals of hydrates such as trans-K4[{Cp*Rh}2Nb6O19]·20H2O (ref. 9) and trans-K4[{Cp*Ir}2Nb6O19]·22H2O (ref. 10) consists of the layers formed by hybrid anions and solvated cations which stack together only through π–π interactions between organic ligands. Methanol can penetrate between such layers and replace water in the coordination spheres of the cations.
Experimental
General procedures
The starting Na7H[Nb6O19]·15H2O was synthesized as described in the literature.17 [(C6H6)RuCl2]2 was obtained from the reaction of RuCl3·xH2O (Krastsvetmet) with 1,3-cyclohexadiene (Sigma Aldrich) by the standard procedure.18 K4[trans-{Cp*Rh}2Nb6O19]·20H2O and K4[trans-{Cp*Ir}2Nb6O19]·22H2O were prepared as described in the literature.9,10 Other reagents were of commercial quality and used as purchased. Methanol was purified according to standard methodology. IR spectra (4000–400 cm−1) were recorded on an IFS-85 Bruker spectrometer.Electrospray ionization (ESI) mass spectra
Electrospray ionization (ESI) mass spectra were recorded on a QTOF Premier (quadrupole-T-wave-time-of-flight) instrument. The temperature of the source block was set to 100 °C and the desolvation temperature to 200 °C. A capillary voltage of 3.3 kV was used in the negative scan mode and the cone voltage was set to 10 V to control the extent of fragmentation of the identified species. Mass calibration was performed with a solution of sodium iodide in isopropanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (50
water (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) in the mass range from m/z 50 to 3000. Sample solutions ca. 5 × 10−5 M in water or methanol were infused via a syringe pump directly connected to the ESI source at a flow rate of 10 μl min−1. The observed isotopic pattern of each compound perfectly matched the theoretical isotope pattern calculated from their elemental composition using the MassLynx 4.1 program.
50) in the mass range from m/z 50 to 3000. Sample solutions ca. 5 × 10−5 M in water or methanol were infused via a syringe pump directly connected to the ESI source at a flow rate of 10 μl min−1. The observed isotopic pattern of each compound perfectly matched the theoretical isotope pattern calculated from their elemental composition using the MassLynx 4.1 program.
NMR experiments
1H and 13C NMR spectra were recorded on a Bruker Avance III 500 spectrometer with inner references in CD3OD at different temperatures.DFT calculations
The experimentally determined geometries of Na7H[Nb6O19]·14H2O (ref. 19) and K4[trans-{Cp*Ir}2Nb6O19]·22H2O (ref. 10) were used as the initial input for the geometry optimization of the anions. The converged molecular structures changed only slightly from the geometries found in the crystal. The structures with coordinated MeO ligands were constructed in silico. Quantum chemical calculations were carried out with the Amsterdam Density Functional (ADF2013.01.c) program (ADF2013, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, the Netherlands, http://www.scm.com). The calculations were performed with an all-electron Slater type TZ2P basis set. As with the geometry optimization, the VWN (local density approximation) and Becke–Perdew (generalized gradient approximation) functionals were used. Relativistic corrections were introduced by both scalar and spin–orbit relativistic zeroth order regular approximation (ZORA).A Hirshfeld population analysis was performed and NMR spectra obtained according to the corresponding modules of the ADF program.
X-ray crystallography
Crystallographic data and refinement details are given in Table S2.† The diffraction data were collected on a Bruker Apex Duo (for 1) and Bruker X8 Apex (for 2 and 3) diffractometers with MoKα radiation (λ = 0.71073 Å) by doing φ and ω scans of narrow (0.5°) frames at 100 K. Structures were solved by direct methods and refined by full-matrix least-squares treatment against |F|2 in an anisotropic approximation in SHELX 2014/7 with a ShelxLe program.20 Absorption corrections were applied empirically with the SADABS program.21 All non-hydrogen atoms of main structural units were refined anisotropically. Positions of all cations in both structures were fully occupied, which excluded models with protonated polyoxoanions. Further details may be obtained from the Cambridge Crystallographic Data Center on quoting the depository numbers CCDC 1423580–1423582.Conclusions
The hybrid complex A4[{LM′}2M6O19] ({LM′} = {Cp*Rh}2+, {Cp*Ir}2+, {(C6H6)Ru}2+; M = Nb, Ta; A = Na, K, Cs) is soluble only in a single organic solvent, namely CH3OH. ESI-MS and NMR data of the methanolic solutions confirm the presence of methoxo complexes [{LM′}2M6O19−n(OCH3)n] (n = 1–3). NMR experiments show that about half of the starting [{LM′}2M6O19]4− is present as methylated derivatives. Crystallization of the solutions of Na4trans-[{(C6H6)Ru}2Nb6O19], K4trans-[{Cp*Rh}2Nb6O19]·20H2O and K4trans-[{Cp*Ir}2Nb6O19]·22H2O in methanol leads to isolation of Na4trans-[{(C6H6)Ru}2Nb6O19]·14.125MeOH·2H2O (1), K4trans-[{Cp*Rh}2Nb6O19]·4MeOH·10H2O (2) and K4trans-[{Cp*Ir}2Nb6O19]·10MeOH·4H2O (3) where direct coordination of CH3OH to Na+ or K+ is observed. The crystal structure of 1 is the first example of a 3D framework built from dimeric [(MeOH)3Na(μ-MeOH)Na(H2O)(MeOH)2]2+ cations and hybrid anions, which is typically new in the case of all known crystal structures of hybrid complexes. Its stability and sorption properties are under investigation.DFT calculations were used for comparison of the 13C NMR shifts of different positions of methoxo ligands in the [{Cp*Ir}Nb6O18(OCH3)]5− backbone. Comparison of the energies of [{Cp*Ir}Nb6O18(μ-OCH3)]5− and [{Cp*Ir}Nb6O18(OCH3)]5− gives very close equal values suggesting that rapid exchange of the MeO− ligand between different positions can be very fast in the solution.
Acknowledgements
This research was supported by Russian Science Foundation (grant number RScF 14-13-00645). The authors are also grateful to the SCIC of the Universitat Jaume I for providing mass spectrometric facilities.Notes and references
- M. Nyman, Dalton Trans., 2011, 40, 8049 RSC; H.-L. Wu, Z.-M. Zhang, Y.-G. Li, X.-L. Wang and E.-B. Wang, CrystEngComm, 2015, 17, 6261 RSC; P. A. Abramov, M. N. Sokolov, S. Floquet, M. Haouas, F. Taulelle, E. Cadot, E. V. Peresypkina, A. V. Virovets, C. Vicent, N. B. Kompankov, A. A. Zhdanov, O. V. Shuvaeva and V. P. Fedin, Inorg. Chem., 2014, 53, 12791 CrossRef CAS PubMed; P. A. Abramov, C. Vicent, N. B. Kompankov, A. L. Gushchin and M. N. Sokolov, Chem. Commun., 2015, 51, 4021 RSC.
- J.-H. Song and W. H. Casey, Chem. Commun., 2015, 51, 12744 RSC; J.-H. Song, D. H. Park, D. A. Keszler and W. H. Casey, Chem.–Eur. J., 2015, 21, 6727 CrossRef PubMed; J.-H. Song, J. R. Wang and W. H. Casey, Dalton Trans., 2014, 43, 17928 RSC; J.-H. Song, C. A. Ohlin, R. L. Johnson, P. Yu and W. H. Casey, Chem.–Eur. J., 2013, 19, 5191 CrossRef PubMed; J.-H. Song, C. A. Ohlin, E. C. Larson, P. Yu and W. H. Casey, Eur. J. Inorg. Chem., 2013, 1748 Search PubMed.
- E. J. Graeber and B. Morosin, Acta Crystallogr., 1977, 33, 2137 CrossRef CAS; E. M. Villa, C. A. Ohlin, E. Balogh, T. M. Anderson, M. D. Nyman and W. H. Casey, Angew. Chem., Int. Ed., 2008, 47, 4844 CrossRef PubMed.
- M. Maekawa, Y. Ozawa and A. Yagasaki, Inorg. Chem., 2006, 45, 9608 CrossRef CAS PubMed.
- M. Matsumoto, Y. Ozawa, A. Yagasaki and Y. Zhe, Inorg. Chem., 2013, 52, 7825 CrossRef CAS PubMed.
- D. Laurencin, R. Thouvenot, K. Boubekeur and A. Proust, Dalton Trans., 2007, 1334 RSC.
- V. W. Day, W. G. Klemperer and C. Schwartz, J. Am. Chem. Soc., 1987, 109, 6030 CrossRef CAS.
- C. Hill and H. Yugi, J. Am. Chem. Soc., 1993, 25, 11823 Search PubMed.
- P. A. Abramov, M. N. Sokolov, A. V. Virovets, S. Floquet, M. Haouas, F. Taulelle, E. Cadot, C. Vicent and V. P. Fedin, Dalton Trans., 2015, 44, 2234 RSC.
- P. A. Abramov, C. Vicent, N. B. Kompankov, A. L. Gushchin and M. N. Sokolov, Eur. J. Inorg. Chem., 2016, 154–160 CrossRef CAS.
- P. A. Abramov, T. P. Zemerova, N. K. Moroz, N. B. Kompankov and M. N. Sokolov, Inorg. Chem., 2016 DOI:10.1021/acs.inorgchem.5b01806.
- W. Clegg, M. R. J. Elsegood, R. J. Errington and J. Havelock, J. Chem. Soc., Dalton Trans., 1996, 681 RSC.
- E. Pretsh, T. Clerc, J. Seibl and W. Simon, Tables of Spectral Data for Structure Determination of Organic Compounds, Springer Verlag, Berlin, 2nd edn, 1989 CrossRef CAS; C. Tsiao, D. Corbin and C. Dybowski, J. Am. Chem. Soc., 1990, 112, 7140 CrossRef CAS; D. Crans, R. Felty, H. Chen and H. Ectert, Inorg. Chem., 1994, 33, 2427 CrossRef.
- A. Karaliota, M. Kamariotaki and D. Hatzipanayioti, Transition Met. Chem., 1997, 22, 411 CrossRef CAS.
- L. Hubert-Pfalzgraf and J. Riess, Inorg. Chim. Acta, 1980, 41, 111 CrossRef CAS; L. Hubert-Pfalzgraf, M. Postel and J. Riess, in Comprehensive Coordination Chemistry Pergamon, ed. G. Wilkinson, Oxford, 1987, vol. 3, pp. 585–698 CrossRef, and ref. therein; L. Hubert-Pfalzgraf, Polyhedron, 1994, 13, 1181 CrossRef; F. A. Cotton, M. Diebold and W. Roth, Inorg. Chem., 1987, 26, 3319 CrossRef; F. A. Cotton, M. Diebold and W. Roth, Inorg. Chem., 1987, 26, 3323 CrossRef.
- R. Tsunashima, D. L. Long, H. Miras, D. Gabb, C. P. Pradeep and L. Cronin, Angew. Chem., Int. Ed., 2010, 49, 113 CrossRef CAS PubMed ; in solid state see for example: L. Jahnberg, J. Solid State Chem., 1970, 1, 454 CrossRef; L. Jahnberg, Mater. Res. Bull., 1981, 16, 513 CrossRef; N. Masó, D. I. Woodward, P. A. Thomas, A. Várez and A. R. West, J. Mater. Chem., 2011, 21, 2715 RSC; R. Hofmann and R. Gruehn, Z. Anorg. Allg. Chem., 1990, 590, 81 CrossRef; J. Busch and R. Gruehn, Z. Anorg. Allg. Chem., 1994, 620, 1066 CrossRef.
- C. M. Flynn and G. D. Stucky, Inorg. Chem., 1969, 8, 178 CrossRef CAS.
- M. A. Bennett, T.-N. Huang, T. W. Matheson and A. K. Smith, Inorg. Synth., 1982, 21, 74 CrossRef CAS.
- A. Goiffon, E. Philippot and M. Maurin, Rev. Chim. Miner, 1980, 17, 466 CAS.
- C. B. Hübschle, G. M. Sheldrick and B. Dittrich, J. Appl. Crystallogr., 2011, 44, 1281 CrossRef PubMed.
- APEX2 (Version 1.08), SAINT (Version 7.03), and SADABS (Version 2.11), Bruker Advanced X-ray Solutions, Bruker AXS Inc., Madison, Wisconsin, USA, 2004 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: ESI-MS data, NMR data, crystal packing information, details of X-ray experiments and refinement. CCDC 1423580–1423582. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra23918d |
| This journal is © The Royal Society of Chemistry 2016 |