Exploration of interactions between decyl-β-d-glucopyranoside and bovine serum albumin in aqueous solution
Abstract
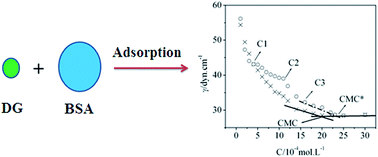
The interactions between decyl-β-D-glucopyranoside (DG) and bovine serum albumin (BSA), in aqueous media, were investigated through the use of surface tension, steady-state fluorescence, and UV-vis absorption spectroscopy measurements. With regard to surface tension, experimental results revealed that the critical micelle concentrations (CMC) within the DG solution, in the absence and presence of BSA, were evaluated as 2.0 mM and 2.34 mM, respectively. Furthermore, the average number of bound DG monomers per BSA molecule was 67 at the critical micelle concentration. Fluorescence and UV-vis absorption spectroscopy indicated that DG had the capacity to quench the intrinsic fluorescence via the formation of DG/BSA complexes. Iodine ion quenching studies have suggested that DG molecules may act to displace tryptophan residues (Trp 214) from their hydrophobic cavities to the surfaces of the protein molecules.

 Please wait while we load your content...
Please wait while we load your content...