An electrokinetic-combined electrochemical study of the glucose electro-oxidation reaction: effect of gold surface energy†
N. Arjona*a,
G. Trejob,
J. Ledesma-Garcíac,
L. G. Arriagab and
M. Guerra-Balcázar*c
aCentro de Investigación y Desarrollo Tecnológico en Electroquímica S. C., Unidad Tijuana, B.C. C.P. 22444, México. E-mail: noe.arjona@yahoo.com.mx; wvelazquez@cideteq.mx; Tel: +52 (664) 6602054 ext. 4416
bCentro de Investigación y Desarrollo Tecnológico en Electroquímica S. C., Parque Tecnológico s/n, Sanfandila, Pedro Escobedo, Qro. C.P. 76703, México
cFacultad de Ingeniería, División de Investigación y Posgrado, Universidad Autónoma de Querétaro, Centro Universitario Cerro de las Campanas, Qro. C.P. 76010, México. E-mail: minbalca@yahoo.com.mx; Tel: +52 (442) 19200 ext. 65421
First published on 29th January 2016
Abstract
The glucose electro-oxidation reaction typically involves several steps and it is strongly influenced by the crystalline structure. In this paper, gold with typical {111} defects (namely Au{111}) and gold with defects enclosed in the (200) plane (Au{200}) were used to determine the effect of the surface energy in the adsorption and electro-oxidation of D-(+)-glucose. To this end, an electrokinetic analysis of surface species was made by means of zeta potential (ζ) measurements and was correlated with an electrochemical study. At low glucose concentration (0.1 mM), the system Au{200} showed a positive and large ζ value of 261.26 mV related to protons from the glucose dehydrogenation. Au{111} presented a negative ζ value of −98.11 mV associated to the glucose chemisorption plus OH− adsorption from the electrolyte. At a higher concentration (>20 mM) both systems exhibited positive ζ values (from 40 to 60 mV) related to the glucose dehydrogenation because of saturation of the electrical double layer by glucose molecules. Through cyclic voltammetry, it was observed that at low glucose concentration (<20 mM), both materials had preference for oxidation of glucose by-products. However, at higher concentrations, Au{111} favors glucono-lactone oxidation (0.4 V vs. NHE); meanwhile Au{200} favors glucose oxidation (−0.43 V vs. NHE). Through the electrokinetic analysis, the behavior of Au{111} can be related to its affinity toward the chemisorption of glucose molecules, and that of Au{200} to weak glucose chemisorption, which allows the desorption of glucose by-products renewing the gold surface for the further oxidation of glucose molecules.
Introduction
Electrocatalysis is a fundamental part of the electrochemical energy conversion areas such as fuel cells, batteries and electrolyzers, and the effect of electrocatalytic materials on their performance needs to be understood in order to upgrade them. These materials are complex systems where the resulting performance such as activity, selectivity and/or stability depends on multiple factors.1 The material facets, surface defects, metal–support interactions, bulk and surface composition, specific surface properties and others are examples of the electrocatalytic complexity.2–7 Koper et al.1 mentioned that the predominant effects can be grouped in to structural or electronic effects. Delgado et al.8 defined the electrokinetic phenomena (EP) as manifestations of the electrical properties of interfaces, which in a practical sense are usually the unique source of information/characterization for those properties. The electrokinetic phenomena are the result from the motion of a pure liquid (or a solution) through a surface where the flow can be driven by an applied potential or by a pressure gradient.9 Typical EP driven by an applied potential are electro-osmosis and electrophoresis. Meanwhile, typical EP resulting from a pressure gradient are streaming potential and streaming current.10 Surfaces in contact with a solution can be electrically charged, and as consequence form an electrical double layer, in two ways: (I) ionization or dissociation of the surface group, and (II) by the surface adsorption of ions in solution.11 These phenomena can be measured by means of the electrical potential (ψ). Typically, the diffuse double layer potential (ψd) is measured by the colloidal probe AFM force technique.12–15 Nevertheless, it can be easily measured by streaming potential or streaming current experiments due to in a practical sense the electrical potential (ψd) and the zeta potential (ζ) showing almost the same value.16The information obtained from the so-called zeta potential (also known as electrokinetic potential or slip plane potential) is probably the best resource to characterize a metal–solution interface. The magnitude of this parameter strongly depends on four variables: (I) the surface nature, (II) its charge (as a function of pH), (III) the electrolyte concentration in the solution and (IV) the nature of the electrolyte.4 Zeta potential measurements usually are obtained to understand the adsorption mechanism of some species as a function of some of the four variables mentioned before.17–19
The mechanism for the glucose electro-oxidation reaction (GOR) has been studied by several authors.20–23 The GOR shows a complex mechanism due to the multiple numbers of by-products which can be formed. Glucose oxidation depends strongly on the electrode nature, surface energy, particle size, pH and others.24 In aqueous media the two most common isomers of D-glucose are α-D-glucose and β-D-glucose in a 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 63 proportion (Scheme 1). Both of these species correspond to the hemi-acetal glucose cyclic form.25 This indicates that glucose in aqueous media is more stable in its cyclic form than in the branched form.
63 proportion (Scheme 1). Both of these species correspond to the hemi-acetal glucose cyclic form.25 This indicates that glucose in aqueous media is more stable in its cyclic form than in the branched form.
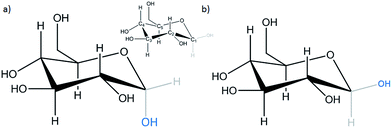 | ||
| Scheme 1 Forms of D-glucose in aqueous media: (a) α-D-glucose and (b) β-D-glucose. Inset: the nomenclature of carbon elements that indicate the glucose molecule. | ||
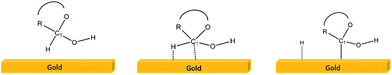
The first step for the electro-oxidation or electro-reduction of any electro-active species (i.e. formic acid, ethanol, methanol, oxygen, etc.) involves its adsorption on a metal surface. According to the Pletcher theory for the concentric adsorption, the electrocatalytic process involves hydrogen extraction followed by simultaneous adsorption of the organic species (Scheme 2, i.e. glucose adsorption on the gold surface).26 Furthermore, in the case of gold electrodes, it is known that the catalytic component is actually the gold hydroxide (AuOH), which is formed by chemisorption of OH− anions on the gold surface. Thus, at alkaline pH this effect is more prominent.27,28
OHads (chemisorbed) has a strong influence on the slow step of glucose electro-oxidation.28 After the adsorption processes, the dehydrogenated glucose molecule can be oxidized to gluconate or to δ-glucono-lactone. It is known that the ratio between these oxidations is influenced by the electrode surface structure. The reaction mechanism, which involves both adsorption and oxidation, strongly depends on the structure; additionally it depends on the glucose concentration and the electrode potential.27,28 The dependence on the surface structure manifests dependence on the adsorption sites and on the surface activity.24 This fact supports the theory of the activation model by chemisorption.
In this work, gold was electrodeposited on glassy carbon electrodes in order to obtain two surfaces with different energies: a gold surface with high (111) defects by cyclic voltammetry and a gold surface with high (200) defects by differential pulse amperometry. D-(+)-Glucose adsorption was evaluated in the presence and absence of potassium hydroxide, which is commonly used as an electrolyte in energy conversion applications, through zeta potential values which were obtained by means of streaming potential/current measurements. The effect of the (111) and (200) defects in the glucose adsorption was correlated with the glucose mechanism and therefore, with their effect in the total electrocatalytic activity by cyclic voltammetry experiments.
Experimental
Electrodeposition of gold on glassy carbon electrodes
The electrodeposition of gold surfaces with (111) and (200) defects was previously reported.29 In brief, a solution composed of 4 mM chloroauric acid (HAuCl4, ≥99%, Sigma Aldrich) as the gold ions source and 0.1 M perchloric acid (HClO4, 69.8%, J. T. Baker) as the electrolyte was prepared and used as the working solution without any surfactant or additive. The electrochemical syntheses were done using a typical glass-jacked three-electrode electrochemical cell through an AutoLab PGSTAT 300 Potentiostat/Galvanostat (Metrohm®). The electrode configuration consisted of glassy carbon plates (20 × 10 mm, SPI® instruments) as the working electrode, a saturated calomel electrode (BAS®, 0.241 V vs. NHE) as the reference, and graphite plates as the counter electrode. All experiments were carried out at 25 °C and with an inert nitrogen atmosphere (99.999%, Infra) and were normalized by the normal hydrogen electrode (NHE). Gold with a high presence of (111) defects (namely Au{111}) was synthesized by means of cyclic voltammetry, using a potential window between 0.183 and 1.583 V vs. NHE with a scan rate of 100 mV s−1 for 20 cycles. Gold with a high content of (200) defects (namely Au{200}) was synthesized by differential pulse amperometry using two pulse potential, a relaxing potential of 1.238 V vs. NHE, and a deposition pulse potential of 0.783 V vs. NHE, with a pulse duration of 0.1 s, and this was repeated for 2500 cycles.Electrokinetic characterization
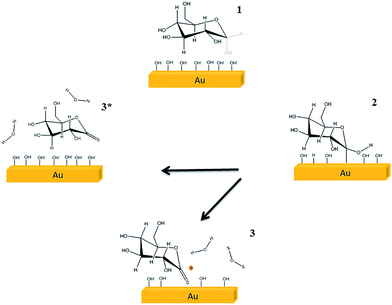
The streaming potential/current values were obtained using the Electrokinetic Analyzer for Solid Surface Analysis: SurPASS (Anton Paar®). The adjustable gap cell was used for all experiments; this cell consists of two parallel plates (20 × 10 mm) where the gap is tuned. The glassy carbon plates were deliberately cut with 20 × 10 mm dimensions in order to fit in the adjustable gap cell. The apparent zeta potential (ζ) was determined following the approximation of the Helmholtz–Smoluchowski equation through streaming current (eqn (1)) and streaming potential (eqn (2)) measurements.30,31 The streaming potential is related to the specific conductivity of the electrolytic solution. The influence of the surface conductance is taken into account and corrected using the Fairbother and Mastin approximation (eqn (3)).32
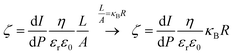 | (1) |
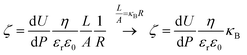 | (2) |
 | (3) |
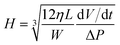 | (4) |
D-(+)-Glucose (reagent degree, Sigma Aldrich) was used for the electrokinetic analysis in the glucose adsorption mechanism. 10 mM potassium hydroxide (87%, J. T. Baker) was used as an electrolyte and hence as the OH− ions source. This concentration was used because at higher concentrations the ionic conductivity strongly affects the streaming current/potential measurements. The potentiometric titrations were made using 0.3 N hydrochloric acid (37%, J. T. Baker) and 0.1 N sodium hydroxide (95%, J. T. Baker). Deionized water (18.4 Ω cm−1, Eccopure®) was further deionized through a Thermo Scientific Barnstead Easypure II®. The final conductivity of the deionized water was 0.2 μS cm−1. The streaming current/potential measurements were done at 400 mbar with an electrode gap of 100 ± 2 μm. The working temperature was 25 °C.
Electrochemical characterization
Cyclic voltammetry was used to investigate the effect of the glucose concentration on the electrocatalytic properties of the Au{111} and Au{200} systems. Cyclic voltammograms were obtained using the same electrochemical configuration as that employed for the electrochemical synthesis. 0.3 M KOH (J. T. Baker, 87%) was used instead of 10 mM KOH to avoid the migration contribution. Cyclic voltammetry in acidic media was performed before and after the glucose electro-oxidation experiments as a function of glucose concentration in order to determine the electrochemical active surface area (ECSA) and hence normalize the cyclic voltammograms. Also, the cyclic voltammograms in acid media were used to corroborate the presence of the {111} and {200} terraces, the physicochemical evidence of the crystallographic planes and geometry as well as reproducibility and geometry stability, which can be found in the ESI (Fig. S1–S3†).Results and discussion
Electrokinetic characterization
The flow rate vs. pressure plots for the system Au{200} with glucose (a) and with glucose + 10 mM KOH (b) are shown in Fig. 1. The system Au{111} exhibited a similar behavior to system Au{200}, and for this reason only the Au{200} system was discussed. A non-linear flow rate behavior was observed in the presence of a lower glucose concentration than 10 mM (Fig. 1a, inset) probably due to the poor dissociation of glucose in water. The flow rate tended to decrease with a quasi-linear behavior at higher concentrations than 20 mM. When an electrolyte with good ionic conductivity and a high pH value such as 10 mM KOH (242.1 mS cm−1, pH > 11) is added to the solution (Fig. 1b), the glucose molecules presented higher dissociation and adsorption on the gold surface (Scheme 2).28 In this context, when the glucose concentration was increased, the flow rate had a tendency to decrease, which is related to the higher counterion migration from the electrical double layer (EDL) to the end of the channel (in the opposite direction to the pressure-driven flow) by effect of the streaming potential (Fig. 1b, inset). Yang et al.34 found that at very low concentrations (in the order of 10−6 and 10−8 M) the flow rate decreases because of the strong effect of the EDL. In our case, higher glucose concentrations were used (10−4 to 10−1 M) where the decrease of the flow rate could be meanly related to ionic-aggregated formation. The EDL effects were depreciated due to the Debye lengths being lower than 30 nm (the electrode gap was 100 μm). | ||
| Fig. 1 Flow rate vs. pressure plots for the system Au{200} as a function of (a) glucose and (b) glucose in the presence of 10 mM KOH as a basic electrolyte. | ||
The OH− adsorption from KOH used as the electrolyte for both gold surfaces and the effect of glucose at low (0.1 mM) and high (100 mM) concentrations on the ζ values of the KOH solution are shown in Fig. 2.
The ζ values in the presence of 10 mM KOH and 0.1 mM glucose for the system A{111} were −56.64 and −22.8 mV, respectively (Table 1 and Fig. 2A). The resulting zeta potential of the 0.1 mM glucose + 10 mM KOH solution was more negative than their separate components probably due to both OH− and glucose adsorption. Increasing the glucose concentration until it reached 100 mM in the KOH solution resulted in a positive ζ value of 40.18 mV (Fig. 2B and Table 1), despite the negative ζ values of 100 mM glucose and 10 mM KOH. For the system labelled as Au{200}, the zeta potential value of glucose in water (Table 1) became more negative by means of increasing the glucose concentration probably due to the higher presence of glucose molecules. In the case of 10 mM KOH as the electrolyte, glucose at 0.1 mM (261.26 mV, Fig. 2C) showed a higher positive zeta potential value than that of 100 mM glucose (46.69 mV, Fig. 2D).
| Electrolyte | ζ Au{111} (mV) | ζ Au{200} (mV) |
|---|---|---|
| 10 mM KOH | −56.64 | −75.75 |
| 0.1 mM glucose | −22.80 | −38.15 |
| 0.1 mM glucose + 10 mM KOH | −98.11 | 261.26 |
| 100 mM glucose | −21.59 | −56.98 |
| 100 mM glucose + 10 mM KOH | 40.18 | 46.69 |
Variations in the zeta potential values as a function of the presence of electrolyte and as a function of glucose at low and high concentrations evidenced changes in the adsorption mechanism, hence the glucose adsorption was investigated in a broader range of glucose concentrations without (Fig. 3a) and with (Fig. 3b) 10 mM KOH as the electrolyte. Meanwhile, the phenomena resulting from Fig. 2 and 3 are illustrated in Scheme 3.
 | ||
| Fig. 3 Effect of the glucose concentration on the zeta potential of Au with {111} and {200} terraces in (a) the absence and (b) the presence of 10 mM KOH. | ||
 | ||
| Scheme 3 Representation of the main phenomena that occur in the Au/solution interface by means of the electrolyte. | ||
From the ζ vs. glucose concentration (Fig. 3a) two things were observed: first, ζ reminded negative in all of the concentration range for both Au{111} and Au{200}; and second, Au{111} is practically insensitive to the addition and increase of glucose concentration (a smooth line was added in Fig. 3a; the observed small variations were related to experimental deviations). Meanwhile for the Au{200} system, the ζ values became more negative with the increase of the glucose concentration. The negative ζ values were related to the oxygen free-electrons (from the hemi-acetal OH, Scheme 1) in the glucose adsorption mechanism (Scheme 3b).35 For Au{200} the increase of the glucose concentration promoted a more negative zeta potential due to the chemisorption of a higher amount of glucose molecules. When the glucose was mixed with 10 mM KOH, as s function of glucose concentration (Fig. 3b) two behaviors were observed: one at low glucose concentrations and another at higher concentrations. At concentrations lower than 5 mM, Au{200} showed positive ζ values which are related to the presence of {200} defects which promotes the glucose adsorption and its further dehydrogenation instead of OH− adsorption. The large positive values were a consequence of the Au{200} affinity to hydrogen adsorption from the breaking of the glucose molecule (Scheme 3c). The Au{111} system showed negative ζ values below 5 mM glucose, which can be related mainly to the OH− adsorption and to the oxygen free-electrons from the hemi-acetal OH in the glucose molecule (Scheme 3d). At higher glucose concentrations than 5 mM both systems showed a similar behavior with positive ζ values. This behavior is related also to the glucose dehydrogenation, where an almost stable positive ζ value was observed due to the saturation of the double layer by effect of the high glucose concentration (>20 mM glucose).
ζ vs. pH curves were obtained at low (1 mM) and high (100 mM) glucose concentrations using 10 mM KOH as the electrolyte in order to evaluate the effect of OH− and H+ ions in the glucose adsorption mechanism (Fig. 4).
Different behaviors were observed at three regions of pH; at high pH (≥11) Au{200} exhibits positive ζ values, meanwhile Au{111} only showed positive values at high glucose concentrations because of the high content of glucose molecules in the metal–surface interface. Also these results are in concordance with those obtained in Fig. 3. A second behavior was observed between the pH values of 4.5 to 10, where both systems exhibited negative ζ values independent of the glucose concentration. This behavior was associated to the strong chemisorption of glucose, where the negative ζ value could be related to the oxygen free-electrons. At pH values below 4.5, both systems (Au{200} and Au{111}) showed positives ζ values related to H+ adsorption. In this region the point of zero charge (PZC) was found at pH values of 4.17 and 4.02 for Au{111} at 1 and 100 mM glucose, respectively. For Au{200} this was found at pH values of 4.45 and 4.31 for 1 and 100 mM glucose. The shift of Au{200} to a relative higher pH than Au{111} could be related to the higher affinity to H+ adsorption than to glucose adsorption.
Electrochemical characterization
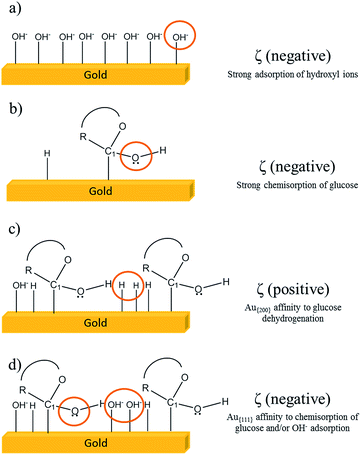
In Fig. 5 the electrochemical profiles of Au{111} and Au{200} are shown. A typical response for Au-based materials was observed. Some differences in the zone of Au oxide formation (1.3 to 1.6 V vs. NHE) were observed, and expected, due to the presence of terraces in the gold surface. Three oxidation peaks were located at 1.36, 1.45 and 1.56 V vs. NHE, respectively (labelled as I, II and III, respectively). The first peak (Fig. 5A and B, peak I) is related to the electro-adsorption of OH− ions;21,36 the second and third peaks (peaks II and III) are related to the removal of OHads in order to form gold oxides, AuO and Au2O3, respectively.37,38 The intensity of the oxidation peaks is related to the specific crystallographic planes,21 and the high intensity of the first and second peaks are related to systems with a high presence of {111} gold terraces.29 Meanwhile, the high intensity of the third peak is characteristic of systems with a high presence of {200} terraces. In this sense, the high presence of {111} and {200} terraces for the systems labeled as Au{111} and Au{200} was corroborated. The system Au{200} showed an intense peak located at 1.06 V, which is attributed to the strong chemisorption of OH− ions on the Au surface. Meanwhile, the same for Au{111} was not observed. This behavior could be explained with the zeta potential results because Au{200} showed a more negative value than Au{111} did for the adsorption of OH− ions. The electrochemical active surface area (ECSA) was determined through these experiments using theoretical charges of 444 and 384 μC cm−2,39 which are characteristic for systems with preferential (111) and (100) planes resulting in areas of 0.8470 and 0.4477 cm2 for Au{111} and Au{200}.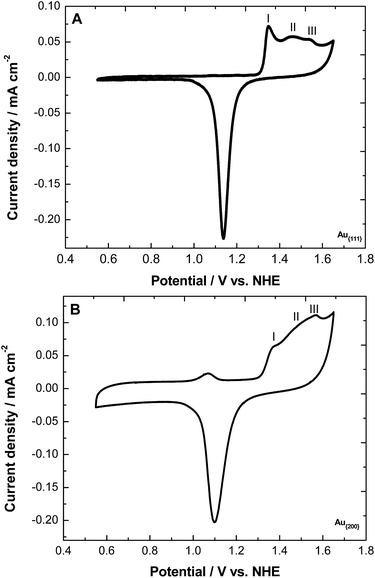 | ||
| Fig. 5 Electrochemical response of Au, (A) with {111} and (B) with {200} terraces in 0.5 M H2SO4. Scan rate: 50 mV s−1. | ||
The evaluation of the electrocatalytic activity of Au{111} and Au{200} surfaces for the glucose electro-oxidation reaction is shown in Fig. 6 and 7, respectively. These graphics are divided into A, B and C for clarity purposes of the effect of glucose concentration. In general, three oxidation peaks were observed and are marked as 1, 2 and 3. The first peak or process (Fig. 6 and 7) is related to the oxidation of glucose,21,29 the second peak/process to the further oxidation of adsorbed lactone, and the third to the formation of a gold oxide layer.
An analysis of the effect of the gold surface on the reaction mechanism can be done through the values of maximum current density of each process. At low glucose concentrations (from 0.1 to 1 mM), the Au{111} surface (Fig. 6A) in the positive sweep shows a 7-fold higher current density of process 2 compared with process 1. Similarly, the Au{200} surface shows, under the same conditions, an 11-fold higher current density for process 2 than process 1. These results indicate that both surfaces at low concentrations tend to the oxidation of glucono-lactone (process 2) instead of glucose (process 1).
The analysis of current densities at the middle concentrations (10 to 40 mM) indicated that the Au{111} surface (Fig. 6B) still preferred to oxidize glucono-lactone. However, the Au{200} surface showed two behaviors. The first was at 10 mM glucose, in which this surface showed a higher current density for process 2 than for process 1. The second was at concentrations of up to 20 mM (Fig. 7B), and for this the current density of process 1 was slightly superior to that of process 2, indicating competition between the glucose and glucono-lactone oxidations. When the concentration was increased to 40 mM, the system Au{200} preferably oxidizes the glucose molecule instead of its by-products. At higher concentrations (Fig. 6C and 7C), Au{111} continued oxidizing glucono-lactone as well as Au{200} still oxidizing glucose instead of its by-products.
An approximation of the glucose electro-oxidation mechanism was done linking both results from the electrokinetic and the electrochemical evaluation (Scheme 4). The mechanism is described as follows: at the beginning, the glucose molecule is deprotonated and adsorbed onto the Au surface (Scheme 4.2). Later, this is oxidized to lactone or glucono-lactone (Scheme 4.3). After that, and according to the electrochemical evaluation, two phenomena can occur: (i) the lactone species is favorably adsorbed on the gold surface, resulting in an increase of the current density of process 2 (Scheme 4.3). And/or (ii) the lactone species is formed and partially removed from the surface (Scheme 4.3*). This capability of glucono-lactone to be removed or stay adsorbed strongly depended on the gold surface. For Au{200}, the high current density of process 1 was related to the removal of glucose by-products because of its ability to dehydrogenate glucose molecules. In this sense, the system is continuously renewed which allows a higher oxidation of glucose as is observed in Fig. 7C. In the case of Au{111}, this surface exhibited affinity to the glucose adsorption (Fig. 2 and Scheme 3), therefore glucose and the glucose by-products are oxidized and remained adsorbed on the Au surface (Scheme 4.3). For this reason, the Au active sites could be blocked avoiding the further oxidation of glucose molecules.
 | ||
| Scheme 4 Limiting steps in the activity of Au-based materials toward the glucose electro-oxidation reaction. | ||
Conclusions
The electrokinetic phenomena are somehow a unique way to characterize the interface between a solid surface and an aqueous electrolyte. Herein, electrokinetic approaches toward glucose adsorption as the first step of the glucose electro-oxidation reaction, and a further electrochemical analysis to the consecutive glucose oxidation steps, were done. For this purpose, two gold surfaces were evaluated: one with defects enclosed in the (111) crystallographic plane and the other in the (200) plane.The adsorption of different electrolytes was analyzed using the determined zeta potential calculated from the streaming current values observing that it was strongly influenced by the gold nature probably due to the different surface energy of the Au{111} and Au{200} systems. D-(+)-Glucose dissolved in water showed negative zeta potential values in both systems due to the adsorption of oxygen free-electrons from the hemi-acetal OH in the glucose molecule. Furthermore, 10 mM potassium hydroxide was used as the electrolyte and glucose as the target molecule concluding that Au with (200) defects showed preference to hydrogen adsorption instead of glucose, where H+ proceeded from the glucose molecule breaking. Meanwhile, Au enclosed in the (111) plane showed preference to the glucose adsorption with and without KOH as the electrolyte. This behavior modified the mechanism of the glucose electro-oxidation reaction: Au{200} preferred the continuous oxidation of glucose molecules, meanwhile Au{111} favored the oxidation of glucose by-products. This can be related with the facility to adsorb or desorb the glucose molecule and the glucose by-products. In summary, the combination of electrokinetic analysis such as the streaming current/potential and electrochemical analysis such as cyclic voltammetry is a powerful tool to infer the reaction mechanism and hence the electrocatalytic activity of electro-active species such as glucose, which is highly employed as fuel in the energy conversion area.
Acknowledgements
The authors gratefully acknowledge the Mexican Council of Science and Technology and the Public Education Ministry for financial support through SEP-CONACYT-2012-01-179921.Notes and references
- A. S. Bandarenka and M. T. M. Koper, J. Catal., 2013, 308, 11–24 CrossRef CAS
.
- R. J. Gillian, D. W. Kirk and S. Thorpe, Electrocatalysis, 2011, 2(1), 1–19 CrossRef
.
- X. Wang, J. Yang, H. Yin, R. Song and Z. Tang, Adv. Mater., 2013, 25, 2728–2732 CrossRef CAS PubMed
.
- S. Zhao, H. Yin, L. Du, G. Yin, Z. Tang and S. Liu, J. Mater. Chem. A, 2014, 2, 3719–3724 CAS
.
- S. Zhao, H. Yin, L. Du, L. He, K. Zhao, L. Chang, G. Yin, H. Zhao, S. Liu and Z. Tang, ACS Nano, 2014, 8, 12660–12668 CrossRef CAS PubMed
.
- H. Yin, H. Tang, D. Wang, Y. Gao and Z. Tang, ACS Nano, 2012, 6, 8288–8297 CrossRef CAS PubMed
.
- D. Strmcnik, K. Kodama, D. Van der Vliet, J. Greeley, V. R. Stamenkovic and N. M. Markovic, Nat. Chem., 2009, 1, 466–472 CrossRef CAS PubMed
.
- A. V. Delgado, F. González-Caballero, R. J. Hunter, L. K. Koopal and J. Lyklema, J. Colloid Interface Sci., 2007, 309(2), 194–224 CrossRef CAS PubMed
.
- G. Hu and D. Li, Chem. Eng. Sci., 2007, 62, 3443–3454 CrossRef CAS
.
- W. B. Zimmerman, Chem. Eng. Sci., 2011, 66(7), 1412–1425 CrossRef CAS
.
- M. Zembala, Adv. Colloid Interface Sci., 2004, 112, 59–92 CrossRef CAS PubMed
.
- J. Drelich, J. Long, Z. Xu, J. H. Masliyah, J. Nalaskowski, R. Beauchamp and Y. Liu, J. Colloid Interface Sci., 2006, 301, 511–522 CrossRef CAS PubMed
.
- M. Borkovec, I. Szilagyi, I. Popa, M. Finessi, P. Sinha, P. Maroni and G. Papastavrou, Adv. Colloid Interface Sci., 2012, 179–182, 85–98 CrossRef CAS PubMed
.
- M. Finessi, I. Szilagyi and P. Maroni, J. Colloid Interface Sci., 2014, 417, 346–355 CrossRef CAS PubMed
.
- A. Filby, M. Plaschke and H. Geckeis, Colloids Surf., A, 2012, 414, 400–414 CrossRef CAS
.
- M. Giesbers, J. Mieke-Kleijn and M. A. Cohen-Stuart, J. Colloid Interface Sci., 2002, 248(1), 88–95 CrossRef CAS PubMed
.
- K. Cai, M. Frant, J. Bossert, G. Hildebrand, K. Liefeith and K. D. Jandt, Colloids Surf., B, 2006, 50(1), 1–8 CrossRef CAS PubMed
.
- E. A. Disalvo and A. M. Bouchet, Colloids Surf., A, 2014, 440, 170–174 CrossRef CAS
.
- M. Markiewicz, W. Mrozik, K. Rezwan, J. Thöming, J. Hupka and C. Jungnickel, Chemosphere, 2013, 90(2), 706–712 CrossRef CAS PubMed
.
- A. Martins, V. Ferreira, A. Queirós, I. Aroso, F. Silva and J. M. Feliu, Electrochem. Commun., 2003, 5, 741–746 CrossRef CAS
.
- M. Pasta, F. La Mantia and Y. Cui, Electrochim. Acta, 2010, 55, 5561–5568 CrossRef CAS
.
- H.-W. Lei, B. Wu, C.-S. Cha and H. Kita, J. Electroanal. Chem., 1995, 382, 103–110 CrossRef
.
- J. Wang, J. Gong, Y. Xiong, J. Yang, Y. Gao, Y. Liu, X. Lu and Z. Tang, Chem. Commun., 2011, 47, 6894–6896 RSC
.
- K. E. Toghill and R. G. Compton, Int. J. Electrochem. Sci., 2010, 5, 1246–1301 CAS
.
- F. Largeaud, K. B. Kokoh, B. Beden and C. Lamy, J. Electroanal. Chem., 1995, 397, 261–269 CrossRef
.
- D. Pletcher, J. Appl. Electrochem., 1984, 14(4), 403–415 CrossRef CAS
.
- L. D. Burke, Electrochim. Acta, 1994, 39(11–12), 1841–1848 CrossRef CAS
.
- Y. B. Vassilyev, O. A. Khazova and N. N. Nikolaeva, J. Electroanal. Chem. Interfacial Electrochem., 1985, 196(1), 127–144 CrossRef
.
- N. Arjona, M. Guerra-Balcázar, G. Trejo, J. Ledesma-García and L. G. Arriaga, New J. Chem., 2012, 36, 2555–2561 RSC
.
- C. Bellmann, A. Synytska, A. Caspari, A. Drechsler and K. Grundke, J. Colloid Interface Sci., 2007, 309, 225–230 CrossRef CAS PubMed
.
- A. Yaroshchuk, E. E. Licón Bernal and T. Luxbacher, J. Colloid Interface Sci., 2013, 410, 195–201 CrossRef CAS PubMed
.
- H. Buksek, T. Luxbacher and I. Petrinic, Acta Chim. Slov., 2010, 57, 700–706 CAS
.
- A. Yaroshchuk and T. Luxbacher, Langmuir, 2010, 26(13), 10882–10889 CrossRef CAS PubMed
.
- C. Yang, D. Li and J. H. Masliyah, Int. J. Heat Mass Transfer, 1998, 41, 4229–4249 CrossRef CAS
.
- S. Ernest, J. Heitbaum and C. H. Hamann, J. Electroanal. Chem., 1979, 100, 173–183 CrossRef
.
- H. Angerstein-Kozlowska, B. E. Conway, B. Barnett and J. Mozota, J. Electroanal. Chem., 1979, 100, 417–446 CrossRef CAS
.
- B. E. Conway, Prog. Surf. Sci., 1995, 49, 331–452 CrossRef CAS
.
- B. Lertanantawong, A. P. O’Mullane, W. Surareungchai, M. Somasundrum, L. D. Burke and A. M. Bond, Langmuir, 2008, 24, 2856–2868 CrossRef CAS PubMed
.
- W. Niu, S. Zheng, D. Wang, X. Liu, H. Li, S. Han, J. Chen, Z. Tang and G. Xu, J. Am. Chem. Soc., 2009, 131, 697–703 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra23780g |
| This journal is © The Royal Society of Chemistry 2016 |