Li4(NH2)3Cl amide-chloride: a new synthesis route, and hydrogen storage kinetic and thermodynamic properties†
Abstract
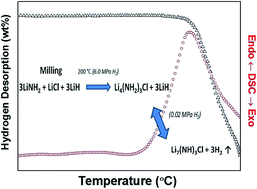
Amide-halide compounds were identified as possible promoters of the dehydrogenation kinetics of the Li–N–H system. However, reversible hydrogen storage capacities and sorption kinetics of Li4(NH2)3Cl and Li3Mg0.5(NH2)3Cl have not been reported yet. In the present work, Li4(NH2)3Cl was synthesized using a new synthesis route that involves the pre-milling of a LiNH2–LiCl mixture. Attempts to synthesize Li3Mg0.5(NH2)3Cl by applying similar synthesis procedures using LiNH2 and 0.5MgCl2 were unsuccessful; instead, a mixture of Li4(NH2)3Cl–0.5Mg(NH2)2 was obtained. The hydrogen storage properties of the Li4(NH2)3Cl–3LiH and Li4(NH2)3Cl–0.5Mg(NH2)2–3LiH composites were evaluated between 200 °C and 300 °C. The onset of hydrogen release was reduced by 20 °C when Li4(NH2)3Cl–3LiH decomposed in the presence of Mg(NH2)2 (180 °C with respect to 200 °C) and its hydrogen desorption rate increased by 83%. However, no change in the dehydrogenation activation energy was observed for Li4(NH2)3Cl–3LiH decomposition due to minor amounts of Mg(NH2)2. The hydrogen storage capacity under cycling was reduced from about 3.0 wt% to 1.5 wt% at 300 °C, after rehydrogenation at 6.0 MPa. The formation of Li7(NH)3Cl was clearly identified in the dehydrogenated material. Unfortunately, the sloped plateau and the thermodynamic stability of Li4(NH2)3Cl–3LiH precludes its hydrogen storage applicability.

 Please wait while we load your content...
Please wait while we load your content...