Oxygen reduction reaction on cobalt–(n)pyrrole clusters from DFT studies†
Abstract
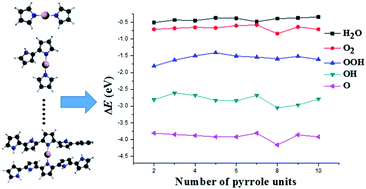
The oxygen reduction reaction (ORR) catalyzed by Co–(n)PPy (n = 2–10) clusters is investigated in detail at BLYP/DZP level of theory. The calculation results indicate that different O2 adsorption modes could greatly affect the types of the reduction intermediates. The side-on O2 adsorption is more likely to yield the intermediate HO–OH, while end-on yields H2O–O. However, the side-on O2 adsorption could lead to strong intra-molecular strain and result in instability of the clusters, the cobalt ion therefore is more easily to be dissolved from the active site, leading to poor durability of the Co–(n)PPy clusters. The ORR activity might be enhanced with the cluster size increases, based on HOMO and LUMO analysis. From Co–(8)PPy, the electronic structures are hard to be modified by simply increasing the PPy chains. However, further increasing the cluster size might result in an increase of Co–N2 active site due to that more Co atoms could be captured by the pyrrolic N atoms, the resulting synergistic effect would be more likely to enhance the activity.

 Please wait while we load your content...
Please wait while we load your content...