A simple method for fabrication of microfluidic paper-based analytical devices and on-device fluid control with a portable corona generator†
Yan Jianga,
Zhenxia Haob,
Qiaohong He*a and
Hengwu Chena
aInstitute of Microanalytical Systems, Department of Chemistry, Zhejiang University, Zijin'gang Campus, Hangzhou 310058, China. E-mail: heqh@zju.edu.cn; Fax: +86-571-88273572; Tel: +86-571-88206773
bTea Research Institute, Chinese Academy of Agricultural Sciences, Hangzhou 310008, China
First published on 22nd December 2015
Abstract
This work presents a facile method for fabrication of microfluidic paper-based analytical devices (μPADs) and on-device fluid control with a portable, hand-held corona generator. First, filter paper was hydrophobized by coupling octadecyltrichlorosilane (OTS) to paper cellulose. Then, the OTS-coated paper, covered with a stenciled plastic mask, was region-selectively exposed to the corona by scanning the electrode tip of the corona generator along the open parts of the mask. Thus, the corona-exposed regions of the paper surface resumed their native hydrophilicity while the masked regions remained highly hydrophobic. The effect of corona treatment time on the paper's hydrophilic property was investigated. Colorimetric assays of nitrite in saliva samples were demonstrated with the developed μPADs. A single-use ‘on’ valve based on wettability-switching was developed in the μPADs. Fluid control was realized via on-site corona discharge targeted at a hydrophobic barrier design as the ‘on’ valve.
1. Introduction
Microfluidic paper-based analytical devices (μPADs), also known as lab-on-paper technology, have attracted great interest since their introduction by Whitesides and coworkers in 2007.1 Advantages of conventional microfluidic chips with the features of paper substrates have emerged and they have thus offered attractive merits since first appearing, including simplicity, inexpensiveness, biocompatibility, portability and disposability. However, the key advantage of μPADs is that aqueous solutions in the channels could be driven by capillary wicking, and thus no external pumps are required. These features make μPADs a fascinating technology for clinical diagnosis, food quality control and environmental monitoring,2–9 especially in less-developed countries and resource-limited situations.μPAD construction is normally based on the principle of generating hydrophilic–hydrophobic pattern on a paper substrate to create millimeter-sized capillary channels. So far, numerous techniques for fabrication of μPADs have been reported, including photolithography,1,10,11 wax patterning,12–17 plasma treating,18 inkjet printing,19,20 inkjet etching,21,22 plotting,23 and some other techniques.24–26 Photolithography was the first established method for fabrication of μPADs. Whitesides and coworkers relied on exposing photoresist-coated paper to UV lights to create hydrophilic channels defined by hydrophobic cured photoresist.1 Our group demonstrated a novel and facile approach for fabrication of μPADs via silanization of paper cellulose followed by region-selective UV irradiation.10 Photolithography was featured in high resolution but limited by complicated fabrication process and expensive equipment required. Wax-patterning made use of cost-effective and easily available wax to form hydrophobic pattern. Wax could be applied to paper by a variety of techniques, such as wax-printing with a printer,12–14 wax-dipping with a metal mask,15 wax-stamping with a metallic stamp,16 and wax-screen-printing with standard emulsion-based screens.17 In these wax-patterning techniques, wax-printing stood out because it allowed mass production with a simple and fast fabrication process. Unfortunately, wax-printers are also expensive. Inkjet printing was an effective alternative to wax-printing.19,20 Inkjet etching had the unique advantage of being able to print reagents directly into testing zones. However, it had the limitation of repeated printing steps required to generate hydrophilic areas.21,22 Nie et al.23 reported a one-step plotting method to fabricate μPADs by simply using permanent markers and metal templates with specific patterns.
Though μPADs have already experienced a quick development in fabrication techniques, limited work has been carried out to develop functional elements for fluid control within μPAD channels. Up to date, efforts on fluid control within channels are mostly based on three-dimensional μPAD (3D-μPAD) construction. Noh and Phillips27 reported controlled wicking of fluids by adjusting the wettability of channels in the z-direction of 3D-μPADs. Martinez et al.28 controlled the movement of fluids by pressing a single-use ‘on’ button to close a small gap between two vertically aligned microfluidic channels so that fluids were able to wick from one channel to the other. Alternatively, Li et al.18 precut the target channel into two parts and manipulated capillary flow by manually separating or joining them.
In this work, we introduce a hand-held corona generator as an effective, inexpensive and portable tool for μPADs fabrication. Corona discharge treatment has been exploited as an efficient surface modification method for microfluidic chips, especially PDMS chips, during chip bonding29 and channel wettability patterning.30,31 To our best knowledge, no work has been reported on using a portable corona generator to build hydrophilic pattern onto paper. Furthermore, we also demonstrate the feasibility of fluid control on μPADs via opening single-use ‘on’ valves by on-site corona discharge treatment.
2. Experimental section
2.1 Materials and apparatus
Octadecyltrichlorosilane (OTS, 95%) was purchased from Acros Organics (Springfield, NJ, USA). Sylgard 184 silicon elastomer kit consisting of a prepolymer base and a curing agent was obtained from Dow Corning (Midland, MI, USA). Polymethylmethacrylate (PMMA) sheet (2 mm in thickness) was purchased from Donchamp Acrylic Co., Ltd (Taixing, Suzhou, China). Filter paper (Model 203 quantitative filter paper) was from Hangzhou Xinhua Paper Industry Co., Ltd (Hangzhou, Zhejiang, China). Human saliva sample was collected from a volunteer after fasting for 2 h. Indicator solution for nitrite assay contained 50 mmol L−1 sulfanilamide, 330 mmol L−1 citric acid and 10 mmol L−1 N-(1-napthyl) ethylenediamine in 80% methanol.A Model BD-20AC corona generator was from Electro-Technic Products Inc. (Chicago, IL, USA). Plastic masks were fabricated by AMCNC-01 carving machine (Amor Electronic Technology Co., Ltd., Guangdong, China). EPSON Perfection V300 Photo desktop scanner was used to scan the images of colorimetric assays performed on μPADs. Water contact angle was measured by JC 2000 C3 (Shanghai Zhongchen Digital Technic Apparatus Co., Ltd, Shanghai, China). The thickness of hydrophilic layer on filter paper was determined by XTL-20 microscope (Shanghai Pudan Optical Instrument Co., Ltd, Shanghai, China). Attenuated total reflectance Fourier transform-infrared (ATR-FT-IR) spectroscopy measurements were conducted on Nicolet Nexus 470 from Nicolet (Madison, WI, USA). X-ray photoelectron spectroscopy (XPS) measurements were carried out on VG ESCALAB MARK II from VG (UK).
2.2 Preparation of mould complex
The mould complex consisted of a top stenciled PMMA mask with channel pattern, a middle PDMS elastic pad and a bottom flat PMMA plate. The pattern of the stenciled mask was first designed with ArtCAM software and then was transformed to a PMMA sheet with the carving machine. A flat PMMA sheet was cut to an appropriate size to act as the hard bottom supporter. The middle elastic PDMS pad in 5 mm thickness was prepared by casting degassed mixture of Sylgard 184 prepolymer base and curing agent (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) against a flat glass.
1) against a flat glass.
2.3 Fabrication of μPADs
The fabrication process is schematically shown in Fig. 1. Filter paper was silanized to hydrophobic in OTS–hexane solution as our previous work reported.10 Briefly, filter paper was cut to an appropriate size and immersed in 0.1% (v/v) OTS–hexane solution for 5 min. Then, the paper pieces were removed from the solution and rinsed sequentially with n-hexane and water, and dried under nitrogen stream. After silanization, the OTS-coated paper was sandwiched by the mould complex as shown in Fig. 1c, and tightly clamped.The corona was adjusted to a relatively low level to produce a stable but soft corona with minimal crackling and sparking. The electrode tip was moved in ∼2 mm proximity to the paper surface to be treated, and was scanned along the open parts of the mask for a reasonable time (depending on the area to be treated). Thus, hydrophilic pattern was built on the hydrophobic OTS-coated paper substrate.
2.4 Nitrite assay on μPADs
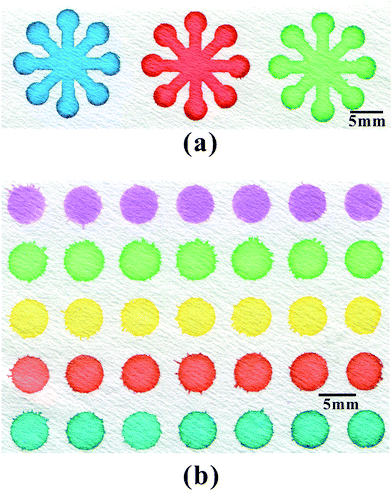
A flower-shaped μPAD with one central reagent zone, eight channels and eight detection zones was fabricated for nitrite assay. The principle of nitrite assay is based on Griess reaction which is a common quantification method for nitrite.32 During the assay, 5 μL of indicator solution was first pipetted into the central reagent zone. The indicator solution penetrated along eight channels into the detection zones by capillary wicking. The device was allowed to air dry under dark conditions for 5 min. Then, 0.20 μL of blank control and serially diluted NO2− standard solutions with the concentration of 0.02, 0.04, 0.08, 0.16, 0.32, 0.64, 1.28 mmol L−1 were individually pipetted into each of the eight detection zones for color development. After standing in dark for 5 min, the μPAD was scanned with a desktop scanner immediately, and the collected image was converted to grayscale with Adobe Photoshop CS3. The assay was repeated three times using three μPADs. The calibration curve was constructed according to the average gray intensities of the standards.Saliva sample was collected and prepared as previously reported.33 Specifically, saliva was collected from a volunteer after fasting for 2 h. Then the sample was centrifuged at 2500 rpm for 10 min at room temperature (15 °C). The supernatant was analyzed for its nitrite contents. When determine the nitrite concentration in saliva sample, a blank control solution and four standard solutions with narrower concentration range (0.02–0.12 mmol L−1) were deposited on 0 and 1–4 zones, respectively, while saliva sample was deposited on 5–7 zones. The nitrite concentration of saliva sample was read against the newly constructed calibration curve based on the standard solutions.
2.5 Safety consideration
The corona generator is safe and easy to use. However some precautions need to be observed. First, when used, the instrument will produce radio frequency which may interfere with other electronic devices such as stopwatch. So they are required be kept at least 1 m away from the generator. Second, the spark emitted from the electrode tip will be easily attracted to metal objects. Thus, metal objects such as knifes should be absent within the operation area, and rings and jewelry should be temporarily removed from the operator. Last, ozone gas is generated around the tip of the electrode when the air is ionized. Therefore, the instrument should be used in areas with good ventilation.3. Results and discussion
3.1 Wettability-patterning of OTS-paper with corona discharge
 | ||
| Fig. 2 The water contact angles measured on the OTS-paper after the paper were exposed to corona discharge with varied time periods. The size of paper sheets: 1 × 3 cm2. n = 9. | ||
The thickness of hydrophilic layer was also measured after the OTS-paper sheets (size: 1 × 0.5 cm2) were exposed to corona discharge for varied time periods. After corona discharge treatment, deionized water colored by green food dye was applied into the paper sheets and allowed air dry for 5 min. Then the dyed paper sheets were cut with a knife to show the smooth cross sections (see Fig. 3a, the thickness of green-colored layer was measured as the thickness of hydrophilic layer. The white layer was hydrophobic and prevented liquid from penetrating through the entire paper thickness). Fig. 3b shows that the thickness of hydrophilic layer increased with the increase of corona discharge treating time until leveled off. Fig. 3b also indicated that the maximum depth corona could reach inside the OTS-paper was ∼50 μm under the experiment conditions.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of carbonyl groups at ∼1723 cm−1 showed up in the spectrum of corona treated OTS-paper (see Fig. S-3c, available in ESI†). This indicated that long alkyl chains of OTS coupling to paper cellulose had been decomposed and oxygen-contained polar groups were introduced into the surface of OTS-paper during corona discharge treatment, resulting in hydrophobic to hydrophilic conversion. It is noticed that the characteristic peaks of cellulose at 1160, 1110, 1056, 1023 cm−1 still existed after corona discharge treatment. This was quite different from the spectrum observed with UV/O3 treated OTS-paper, where all these characteristic peaks of cellulose disappeared.10 It indicated that corona discharge treatment was a mild technique compared to UV/O3 technique, the latter decomposed not only long alkyl chains of OTS but also the skeleton structures of cellulose, consequently, turned the paper from white to yellow-dish. This was the reason why the limit of detection was significantly lowered when the μPADs fabricated by the present method were used in colorimetric analysis.
O stretching of carbonyl groups at ∼1723 cm−1 showed up in the spectrum of corona treated OTS-paper (see Fig. S-3c, available in ESI†). This indicated that long alkyl chains of OTS coupling to paper cellulose had been decomposed and oxygen-contained polar groups were introduced into the surface of OTS-paper during corona discharge treatment, resulting in hydrophobic to hydrophilic conversion. It is noticed that the characteristic peaks of cellulose at 1160, 1110, 1056, 1023 cm−1 still existed after corona discharge treatment. This was quite different from the spectrum observed with UV/O3 treated OTS-paper, where all these characteristic peaks of cellulose disappeared.10 It indicated that corona discharge treatment was a mild technique compared to UV/O3 technique, the latter decomposed not only long alkyl chains of OTS but also the skeleton structures of cellulose, consequently, turned the paper from white to yellow-dish. This was the reason why the limit of detection was significantly lowered when the μPADs fabricated by the present method were used in colorimetric analysis.3.2 Nitrite assay in saliva sample
Saliva contains components derived from serum34 and can be collected more easily and noninvasively than blood. Therefore, saliva has been suggested to be a good alternative to blood for diagnostic purposes. Saliva nitrite has been reported to be a useful biomarker for real-time monitoring of dialysis progression.34,35To exploit the μPADs fabricated by the present technique to clinical diagnosis, colorimetric test of saliva nitrite was conducted on the prepared μPADs. A flower-shaped channel network with eight detection zones was chosen as the device design to perform multi-sample assays simultaneously and under the same condition. Fig. 5a shows the scanned image of a color-developed μPAD with one detection zone for blank control and seven detection zones for serially diluted NO2−. A calibration curve was constructed based on the color intensities of seven detection zones as shown in Fig. 5b. The dynamic linear range for NO2− was 20–160 μmol L−1 (linear regression equation: y = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 123x − 151.7, x for NO2− concentration at millimolar, y for the color intensity; correlation coefficient: 0.9927). The limit of detection defined as the concentration equivalent to 3-folds of standard deviation of color intensities for 3 blank samples was 7.8 μmol L−1, which was lowered by an order of magnitude compared to our previous work.10 This was due to the corona discharge treated paper in the present work remained colorless while the UV/O3-treated paper in the previous work turned slightly yellowish, which resulted in relatively high blank background.
123x − 151.7, x for NO2− concentration at millimolar, y for the color intensity; correlation coefficient: 0.9927). The limit of detection defined as the concentration equivalent to 3-folds of standard deviation of color intensities for 3 blank samples was 7.8 μmol L−1, which was lowered by an order of magnitude compared to our previous work.10 This was due to the corona discharge treated paper in the present work remained colorless while the UV/O3-treated paper in the previous work turned slightly yellowish, which resulted in relatively high blank background.
The NO2− content in saliva sample determined by the proposed method was 68.5 ± 5.1 μmol L−1 (n = 3), which agreed well with the concentration of 69.7 ± 0.2 μmol L−1 (n = 3) determined by ion chromatography. This result demonstrates the feasibility of μPADs fabricated by the proposed method to be a simple and inexpensive approach for de-centralized clinical diagnosis.
3.3 Fluids control on the μPAD via on-site corona discharge treatment
Since the hydrophilization of OTS-paper could be quickly conducted on-site with a hand-held corona generator, it would be possible to prepare a type of single-use ‘on’ valves in μPADs. The valve was designed as a 1 mm wide hydrophobic dam at the end of a channel where the fluid should be temporally stopped. At the moment when the blocked fluid needed to move forward, the valve could be quickly open via targeted corona discharge, then fluid could flow forward again via capillary wicking. To test the feasibility of the proposed single-use ‘on’ valves, a programmed acid–base neutralization process was designed as the model for fluid control, and the μPAD shown in Fig. 6a was fabricated for the test. The device consisted of two sample reservoirs (S1 for basic solution and S2 for acid solution), one reaction cell (R), and two single-use ‘on’ valves (V1, V2) that were positioned at the ends of basic and acid channels, respectively. With such a device, both the acid solution and the basic solution can be programmed to enter the reaction cell by sequentially opening the valves via on-site targeted corona discharge treatment. In order to visualize clearly the results of the programmed acid–base reaction, a small quantity of acid–base indicator (phenolphthalein) solution was applied into the reaction cell. NaOH and HCl solutions were then introduced into sample reservoirs S1 and S2, respectively (Fig. 6a). Both the acid and alkaline solutions were driven forward along the channels by capillary wicking until they were blocked by the closed valves. Afterwards, the electrode tip with soft corona was targeted to the valve V1 for 1 s (Fig. 6b), leading to the valve open. As a result, NaOH solution penetrated into the reaction cell, and the phenolphthalein indicator in the cell gradually turned violet (Fig. 6c and d). Then same operation was done to open V2, consequently HCl solution entered into the reaction cell (Fig. 6e). HCl solution neutralized NaOH solution in the cell. Thus, the alkaline medium in the cell was gradually transformed to acidic, and violet color of indicator faded (Fig. 6f and g). A video recording the whole process was given in ESI.† This programmed acid–base reaction model demonstrated that the closed wettability-switching valves can be instantaneously opened by targeted corona discharge and that an array of such single-use ‘on’ valves can be easily integrated in the channel network to perform complicated fluid control in the μPADs.4. Conclusion
This article has developed the technique for fabricating microfluidic paper-based analytical devices and on-device fluid control by using a hand-held corona generator. The established method features simplicity in operation, low in cost, and less in environmental pollution. Moreover, it allows analysts to fabricate μPADs in their own laboratories. The demonstrated simple approach for salivary nitrite analysis with the developed μPADs may be used as a noninvasive method for point of care tests. The targeted corona discharge can effectively open the wettability-switching valves, and the single-use ‘on’ valves can be easily integrated in the channel network to perform complicated fluid control in the μPADs, which will broaden the practical applications of μPADs for multi-step assays (for instance, immunoassays) that required sequential mixing of reagents and analytes.Acknowledgements
This work is funded by the National Natural Science Foundation of China (Project No. 20890020) and the National Science & Technology Supporting Program of China (Grant 2012BAI13B06).References
- A. W. Martinez, S. T. Phillips, M. J. Butte and G. M. Whitesides, Angew. Chem., Int. Ed., 2007, 46, 1318–1320 CrossRef CAS.
- D. D. Liana, B. Raguse, J. J. Gooding and E. Chow, Sensors, 2012, 12, 11505–11526 CrossRef CAS PubMed.
- D. M. Cate, J. A. Adkins, J. Mettakoonpitak and C. S. Henry, Anal. Chem., 2015, 87, 19–41 CrossRef CAS PubMed.
- X. Li, D. R. Ballerini and W. Shen, Biomicrofluidics, 2012, 6, 11301–1130113 CrossRef PubMed.
- Y. Jiang, C. Ma, X. Hu and Q. He, Prog. Chem., 2014, 26, 167–177 CAS.
- X. Chen, J. Chen, F. Wang, X. Xiang, M. Luo, X. Ji and Z. He, Biosens. Bioelectron., 2012, 35, 363–368 CrossRef CAS PubMed.
- S. Ge, L. Ge, M. Yan, X. Song, J. Yu and J. Huang, Chem. Commun., 2012, 48, 9397–9399 RSC.
- L. Ge, S. Wang, X. Song, S. Ge and J. Yu, Lab Chip, 2012, 12, 3150–3158 RSC.
- X. Hu, L. Lu, C. Fang, B. Duan and Z. Zhu, J. Agric. Food Chem., 2015, 63, 9863–9868 CrossRef CAS PubMed.
- Q. He, C. Ma, X. Hu and H. Chen, Anal. Chem., 2013, 85, 1327–1331 CrossRef CAS PubMed.
- P. D. Haller, C. A. Flowers and M. Gupta, Soft Matter, 2011, 7, 2428–2432 RSC.
- E. Carrilho, A. W. Martinez and G. M. Whitesides, Anal. Chem., 2009, 81, 7091–7095 CrossRef CAS PubMed.
- Y. Lu, W. Shi, L. Jiang, J. Qin and B. Lin, Electrophoresis, 2009, 30, 1497–1500 CrossRef CAS PubMed.
- Y. Lu, W. Shi, J. Qin and B. Lin, Anal. Chem., 2009, 82, 329–335 CrossRef PubMed.
- T. Songjaroen, W. Dungchai, O. Chailapakul and W. Laiwattanapaisal, Talanta, 2011, 85, 2587–2593 CrossRef CAS PubMed.
- P. de Tarso Garcia, T. M. G. Cardoso, C. D. Garcia, E. Carrilho and W. K. T. Coltro, RSC Adv., 2014, 4, 37637–37644 RSC.
- W. Dungchai, O. Chailapakul and C. S. Henry, Analyst, 2011, 136, 77–82 RSC.
- X. Li, J. Tian, T. Nguyen and W. Shen, Anal. Chem., 2008, 80, 9131–9134 CrossRef CAS PubMed.
- X. Li, J. Tian, G. Garnier and W. Shen, Colloids Surf., B, 2010, 76, 564–570 CrossRef CAS PubMed.
- C. Xu, L. Cai, M. Zhong and S. Zheng, RSC Adv., 2015, 5, 4770–4773 RSC.
- K. Abe, K. Suzuki and D. Citterio, Anal. Chem., 2008, 80, 6928–6934 CrossRef CAS.
- K. Abe, K. Kotera, K. Suzuki and D. Citterio, Anal. Bioanal. Chem., 2010, 398, 885–893 CrossRef CAS PubMed.
- J. Nie, Y. Zhang, L. Lin, C. Zhou, S. Li, L. Zhang and J. Li, Anal. Chem., 2012, 84, 6331–6335 CrossRef CAS PubMed.
- J. Olkkonen, K. Lehtinen and T. Erho, Anal. Chem., 2010, 82, 10246–10250 CrossRef CAS PubMed.
- G. Chitnis, Z. Ding, C.-L. Chang, C. A. Savran and B. Ziaie, Lab Chip, 2011, 11, 1161–1165 RSC.
- L. Cai, Y. Wang, Y. Wu, C. Xu, M. Zhong, H. Lai and J. Huang, Analyst, 2014, 139, 4593–4598 RSC.
- H. Noh and S. T. Phillips, Anal. Chem., 2010, 82, 8071–8078 CrossRef CAS PubMed.
- A. W. Martinez, S. T. Phillips, Z. Nie, C. M. Cheng, E. Carrilho, B. J. Wiley and G. M. Whitesides, Lab Chip, 2010, 10, 2499–2504 RSC.
- K. Haubert, T. Drier and D. Beebe, Lab Chip, 2006, 6, 1548–1549 RSC.
- L. A. Filla, D. C. Kirkpatrick and R. S. Martin, Anal. Chem., 2011, 83, 5996–6003 CrossRef PubMed.
- R. T. Davies, D. Kim and J. Park, J. Micromech. Microeng., 2012, 22, 055003 CrossRef.
- X. Li, J. Tian and W. Shen, Anal. Bioanal. Chem., 2010, 396, 495–501 CrossRef CAS PubMed.
- Y. Tanaka, N. Naruishi, H. Fukuya, J. Sakata, K. Saito and S.-I. Wakida, J. Chromatogr. A, 2004, 1051, 193–197 CrossRef CAS PubMed.
- T. M. Blicharz, D. M. Rissin, M. Bowden, R. B. Hayman, C. DiCesare, J. S. Bhatia, N. Grand-Pierre, W. L. Siqueira, E. J. Helmerhorst, J. Loscalzo, F. G. Oppenheim and D. R. Walt, Clin. Chem., 2008, 54, 1473–1480 CAS.
- S. A. Klasner, A. K. Price, K. W. Hoeman, R. S. Wilson, K. J. Bell and C. T. Culbertson, Anal. Bioanal. Chem., 2010, 397, 1821–1829 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra23470k |
| This journal is © The Royal Society of Chemistry 2016 |