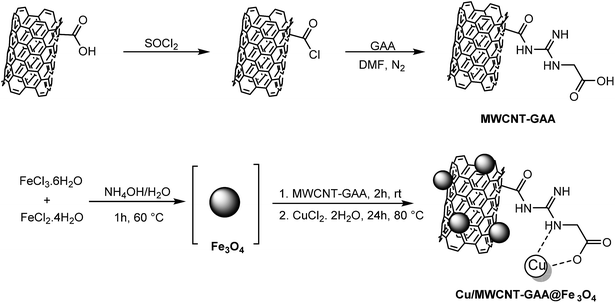
Copper supported on MWCNT-guanidine acetic acid@Fe3O4: synthesis, characterization and application as a novel multi-task nanocatalyst for preparation of triazoles and bis(indolyl)methanes in water†
Ahmad Shaabani*,
Ronak Afshari,
Seyyed Emad Hooshmand,
Azadeh Tavousi Tabatabaei and
Fatemeh Hajishaabanha
Faculty of Chemistry, Shahid Beheshti University, GC, PO Box 19396-4716, Tehran, Iran. E-mail: a-shaabani@sbu.ac.ir
First published on 2nd February 2016
Abstract
The synthesis of a new supported copper (Cu) nanocatalyst, with highly dispersed particles, based on magnetic guanidine acetic acid (GAA) functionalized multi-wall carbon nanotubes (MWCNT), Cu/MWCNT-GAA@Fe3O4, is reported. The synthesized nanocatalyst was characterized by means of X-ray diffraction, scanning electron microscopy, transmission electron microscopy, energy dispersive X-ray, elemental analysis (carbon, hydrogen, nitrogen), thermogravimetric analysis and Fourier transform-infrared. The catalytic activity of Cu/MWCNT-GAA@Fe3O4 was investigated in 1,3-dipolar cycloaddition and condensation reactions in water. The results showed a wide range of 1,2,3-triazoles and bis(indolyl)methanes were synthesized with good to excellent yields in a short time. The nanocatalyst could be recovered by applying an external magnetic field, which resulted in easy separation of the catalyst without filtration and this could be reused for several runs without loss of significant activity.
1. Introduction
Carbon materials such as carbon nanotubes (CNTs) and graphene oxides demonstrate great potential as promising substitutes for conventional catalyst supports because of their large surface area and outstanding electronic conductivity.1–4 Catalysis by metal-based nanocomposites is also a powerful method of accelerating a large number of organic reactions. Very recently, the heterogenization of metals onto a support gained increasing attention in the development of sustainable organometallic chemistry.5 In this respect, CNTs are one of the main choices for the preparation of multi-task, heterogeneous metallic-based catalysts, which are a novel class of catalysts that are able to be involved in at least two consecutive reactions having different mechanisms because of their considerable advantages. However, the limited solubility of CNTs has severely impacted their use, therefore functionalization must be developed for improving their solubility, by efficiently introducing chemically active sites for use in catalytic reactions and which will also act as anchoring sites for deposition of metal nanoparticles (NPs).6,7 Nowadays, the most promising materials for functionalization of CNTs are polymers such as DNA/RNA,8 polyporphyrin9 and chitosan10,11 that can decorate the CNT surface to improve their insolubility. Furthermore, immobilized magnetic molecular complexes or NPs on a support can be recovered from the reaction mixture by use of an external magnet and their recovery and reuse are an additional benefit.12 Additionally, the nanometer size range of these particles facilitates the catalysis process, as an increased surface area is available for the reactions.13The vast majority of the novel heterogeneous catalysts are based on CNT supports, because the organic groups can be robustly anchored to the CNT surface to provide catalytic centers via metal–ligand cooperation. Among the variety of catalytically active metal complexes, the transition metals bearing N, O-donor ligands are of great interest and several papers have reported their catalytic activity in various catalytic organic reactions.14,15 One of these N, O-donor ligands is guanidine acetic acid (GAA). GAA is the essential precursor of creatine, and belongs to the class of guanidino compounds which are characterized by the presence of a basic guanidino group, HN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(NH2)–NH–. Because of its unique properties,16 this amino acid is involved in many important biological processes, such as creatine deficiency,17 renal metabolism,18 thyroid dysfunction,19 epileptic seizures, hepatic encephalopathy and insulin regulation.18 Also, the complexation of GAA with copper [Cu(II)] for its role in superoxide dismutase was studied.20 Therefore, GAA with the N, O-donor ability as a bifunctional ligand can be used to create a novel class of heterogeneous catalysts that can have good properties.
C(NH2)–NH–. Because of its unique properties,16 this amino acid is involved in many important biological processes, such as creatine deficiency,17 renal metabolism,18 thyroid dysfunction,19 epileptic seizures, hepatic encephalopathy and insulin regulation.18 Also, the complexation of GAA with copper [Cu(II)] for its role in superoxide dismutase was studied.20 Therefore, GAA with the N, O-donor ability as a bifunctional ligand can be used to create a novel class of heterogeneous catalysts that can have good properties.
As “point and shoot” coupling tools, click reactions have been appealing to researchers since their discovery,21 and have been widely used in many current research fields. However, because of the high activation energy required, these cycloadditions generally need high temperatures and long reaction times and usually give a mixture of the 1,4- and 1,5-regioisomers. The copper catalysts could facilitate the cycloaddition in a regiospecific manner to give only 1,4-disubstituted triazoles and this was reported by Kolb et al.22 This reaction has been reported using the catalysts in the form of Cu(I) salts,23 a copper sulfate-ascorbate system,24 immobilized Cu(I) on polymers,25 copper NPs,26 and nanostructured copper oxide.27 Therefore, it was inferred that a heterogeneous magnetic Cu/multi-wall carbon nanotube (MWCNT) catalyst system, in the presence of GAA as the ligand, Cu/MWCNT-GAA@Fe3O4, could catalyse this reaction and give rise to click chemistry. Furthermore, for investigating the multi-task capability of a new synthesized nanocatalyst, the catalytic activity was examined in a condensation reaction and interestingly has also been found to be an efficient and reliable catalyst for the simple synthesis of bis(indolyl)methanes in water (H2O) which represents an active area of investigation in pharmaceutical and organic synthesis. Bis(indolyl)methanes, which contain two indole units in a molecule, are found in various natural products and they possess important biological activity28,29 and as an important component have diverse pharmaceutical properties.30,31 One of the most applied procedures for the synthesis of bis(indolyl)methanes is based on the electrophilic substitution reaction of indoles with various aldehydes and ketones in the presence of heterogeneous and homogeneous acid catalysts.32–36
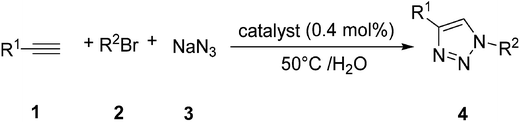
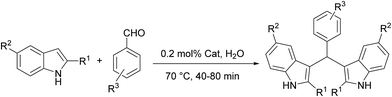
As part of an ongoing program related to the developing new heterogeneous catalysts for organic transformations,37–40 and based on previous investigations on graphene,41–45 in this paper is reported the synthesis and characterization of a new supported Cu nanocatalyst on magnetic GAA functionalized MWCNT (Cu/MWCNT-GAA@Fe3O4). The as-prepared nanocatalyst showed a high catalytic activity towards azide-alkyne 1,3-dipolar cycloaddition reactions for the synthesis of 1,2,3-triazoles and condensation reaction for the synthesis of bis(indolyl)methanes in the presence of H2O as a green solvent, yielding a catalytic efficiency over several cycles (Scheme 1).
 | ||
| Scheme 1 Synthesis of 1,2,3 triazole and bis(indolyl)methane derivatives using a Cu/MWCNT-GAA@Fe3O4 catalyst. | ||
2. Materials and methods
2.1. General
The materials were purchased from Merck and Aldrich and used without further purification. Carboxyl MWCNTs (purity> 95%, –COOH content: 2.00 wt%, outer diameter: 10–20 nm, inner diameter: 5–10 nm, length: 30 μm, special surface area: 220 m2 g−1) was purchased from Atoor Sanat Abtin, Tehran, Iran. Products were analyzed using gas chromatography (GC), Varian 3900 GC. The X-ray diffraction (XRD) pattern of the catalyst was recorded on a diffractometer (STOE & CIE STADI P) with a scintillation detector, and a secondary monochromator using Cu Kα radiation (λ = 0.1540 nm). Copper(II) determination was carried out on an flame atomic absorption spectrometer (FAAS, Shimadzu AA-680) with a Cu hollow cathode lamp at 324.7 nm, using an air–acetylene flame. Transmission electron microscopy (TEM) was performed using a transmission microscope (Philips CM30) with an accelerating voltage of 150 kV. Scanning electron microscopy (SEM) observations were carried out using an environmental scanning electron microscope (Philips XL30 ESEM). Thermogravimetric analysis (TGA) was carried out using a simultaneous thermal analyser (Scinco STA 1500) at a heating rate of 10 °C min−1 in air. Melting points (mp) were measured on a melting point instrument (Electrothermal IA9100) and are uncorrected. Infrared (IR) spectra were recorded on a spectrometer (Shimadzu IR470). The elemental analyses were performed with an Elementar Analysensysteme GmbH Vario EL. Proton (1H) and carbon-13 (13C) nuclear magnetic resonance spectroscopy (NMR) spectra were recorded on a spectrometer (Bruker Avance DRX300) at 300.13 and 75.47 MHz. The NMR spectra were obtained in deuterated dimethylsulfoxide (DMSO-d6) and deuterated chloroform (CDCl3).2.2. Preparation of the catalyst
2.3. General procedure for the synthesis of 1,2,3-triazoles
Alkynes (1 mmol), benzyl bromide derivatives (1 mmol) and sodium azide (1 mmol, 0.065 g) were added to a suspension of catalyst (0.400 mol%, 0.020 g) in H2O (5 mL) in a 10 mL round ringed flask fitted with a magnetic stirrer. The resultant mixture was heated at 50 °C. The progress of the reaction was followed using thin layer chromatography (TLC) (ethyl acetate/n-hexane). Upon completion, the mixture was cooled to room temperature and the catalyst separated using an external magnet and the product was extracted with chloroform. The solvent was removed under vacuum to give the pure product. If necessary, the purification was performed by recrystallization from ethyl acetate or ethanol.2.4. General procedure for the synthesis of bis(indolyl)methanes
An indole (1 mmol), an aldehyde (0.5 mmol) and H2O (5 mL) were placed in a 10 mL round bottomed flask equipped with magnetic stirring bar. The catalyst (0.200 mol%, 0.01 g) was added and the mixture was stirred at 70 °C for 40–80 min. The progress of the reaction was monitored using TLC (ethyl acetate/n-hexane). Then chloroform (2 × 5 mL) was added to the reaction mixture and the catalyst was separated using an external magnet. The chloroform layer was separated, evaporated under reduced pressure to give the crude product which was purified via recrystallization in an ethanol–H2O mixture.3. Results and discussion
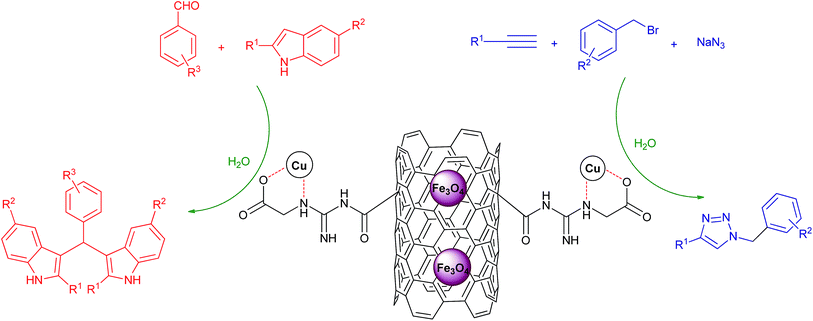
After synthesis of the novel catalyst, Cu NPs were anchored to the side walls of MWCNT using GAA as a linker.47 The schematic pathway for the preparation of Cu/MWCNT-GAA@Fe3O4 is shown in Scheme 2.Cu/MWCNT-GAA@Fe3O4 was prepared by chemical functionalization of MWCNT with GAA and the GAA content in the nanocatalyst was calculated to be 2.62 mmol g−1, according to the N2 content which was determined using carbon, hydrogen, N2 analysis. The FAAS method was used for determining Cu content of the nanocatalyst and the weight percentage of Cu was 1.2%. Fourier-transform-infrared (FT-IR) spectra of the MWCNT-COOH, MWCNT-COCl, MWCNT-GAA and Cu/MWCNT-GAA@Fe3O4 are shown in Fig. 1. The FT-IR peaks for MWCNT-COOH show a broad peak at 3443 cm−1 because of the stretching vibration of the –OH bond, the 1720 cm−1 peak is attributed to the presence of carbonyl groups and the peak at 1382 cm−1 corresponds to the O–H bond in the carboxylic group. A peak at 1221 cm−1 is assigned to the C–O bond stretching (Fig. 1(I-a)). The FT-IR spectra of MWCNT-COCl confirmed the conversion of C–OH groups to C–Cl by the absence of a broad peak of –OH and the presence of a sharp peak at 801 cm−1 which indicated C–Cl bonding and the slight shift in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching to 1729 cm−1 (Fig. 1(I-b)). Immobilization of the GAA on the MWCNT-COCl can be inferred (Fig. 1(II-c)), and, as a result, the carboxylic bonds of CNT have been converted into amide bonds (NH–C
O stretching to 1729 cm−1 (Fig. 1(I-b)). Immobilization of the GAA on the MWCNT-COCl can be inferred (Fig. 1(II-c)), and, as a result, the carboxylic bonds of CNT have been converted into amide bonds (NH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). The interaction is indicated by the disappearance of the C–Cl peak and the appearance of the C–N stretching at 1394 cm−1. In addition, the FT-IR spectrum of MWCNT-GAA has some bands at 3363, 2935 and 1692 cm−1 which correspond to the N–H, C–H, C
O). The interaction is indicated by the disappearance of the C–Cl peak and the appearance of the C–N stretching at 1394 cm−1. In addition, the FT-IR spectrum of MWCNT-GAA has some bands at 3363, 2935 and 1692 cm−1 which correspond to the N–H, C–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretches, respectively, and which were not observed for CNT. Finally, the FT-IR spectrum of Cu/MWCNT-GAA@Fe3O4 exhibits a broad band at 580 cm−1 which corresponds to the stretching vibration of the Fe–O bonds which is assigned to the spinel form of iron oxide (Fe3O4).48 The slight shift in the C
O stretches, respectively, and which were not observed for CNT. Finally, the FT-IR spectrum of Cu/MWCNT-GAA@Fe3O4 exhibits a broad band at 580 cm−1 which corresponds to the stretching vibration of the Fe–O bonds which is assigned to the spinel form of iron oxide (Fe3O4).48 The slight shift in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching bonds to 1620 and 1577 cm−1 may be because of the chelation of Cu NPs on the surface of MWCNT-GAA@Fe3O4 (Fig. 1(II-d)).
O stretching bonds to 1620 and 1577 cm−1 may be because of the chelation of Cu NPs on the surface of MWCNT-GAA@Fe3O4 (Fig. 1(II-d)).
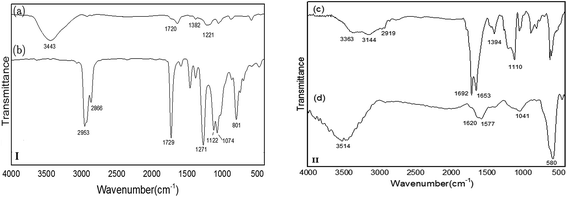 | ||
| Fig. 1 FT-IR spectra of: (I-a) MWCNT-COOH; (I-b) MWCNT-COCl; (II-c) MWCNT-GAA and (II-d) Cu/MWCNT-GAA@Fe3O4. | ||
Selected images obtained using SEM and TEM were used to study the structure and morphology of the catalyst. The SEM and TEM images of MWCNT-COCl show the nanotube structure of this particle with low dispersity (Fig. 2a and d). The shape and structure of MWCNTs remained constant after functionalization with GAA and caused the higher dispersion of nanotubes (Fig. 2e). After functionalization of the MWCNT-COCl via GAA and immobilization of Cu and Fe3O4 NPs, the ligand and immobilized NPs could act as an obstacle in agglomeration of the nanocatalyst so that it could be used as a suitable catalyst for any reaction. To confirm the formation of Cu NPs through the preparation of the nanocatalyst, the Cu/MWCNT-GAA was synthesized by a similar procedure in the absence of Fe2+ and Fe3+. The white dots represent anchored Cu NPs, with a spherical-like shape, and a diameter of ∼20 nm, that were dispersed over the side walls of MWCNT-GAA which could be directly visualized from the SEM and TEM images (Fig. 2b and f). The SEM and TEM images of Cu/MWCNT-GAA@Fe3O4 show that Fe3O4 NPs are incorporated satisfactorily on the surface of Cu/MWCNT-GAA with semi-spherical morphology. Also, Cu NPs can easily be recognized as they appear brighter than Fe3O4 NPs in the TEM image (Fig. 2c and g). The NPs attached to the side walls of the MWCNT-GAA with an average size of 8–25 nm, related to the Fe3O4 and Cu NPs. The components of Cu/MWCNT-GAA and Cu/MWCNT-GAA@Fe3O4 were determined by using energy dispersive (EDX) spectrometry. The EDX spectrum shows the elemental composition (Cu) of Cu/MWCNT-GAA (Fig. 2h) and (O, Fe and Cu) of Cu/MWCNT-GAA@Fe3O4 (Fig. 2i).
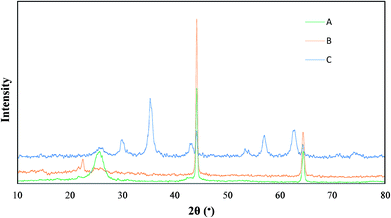
The XRD patterns of MWCNT-COCl, MWCNT-GAA and Cu/MWCNT-GAA@Fe3O4 are shown in Fig. 3. The diffraction peaks at 2θ = 25.53° and 42.28° were associated with (002), (100) corresponding to the hexagonal graphite structure of MWCNT that appeared in all the patterns. For MWCNT-GAA there is no obvious change in the structure of MWCNT after functionalization with GAA. After formation of Cu and Fe3O4 NPs on the surface of functionalized MWCNT, the pattern shows the new peaks. The peaks at 43.3°, 50.26° and 74.56° were assigned to the (111), (200) and (220) demonstrating the formation of Cu NPs,49 also the 30.87°, 35.30°, 42.93°, 53.35°, 57.00°, and 62.66° peaks were obtained, which corresponded to the (220), (311), (400), (422), (511), and (440) because of the cubic spinel structure of the Fe3O4 NPs.50 In brief, these XRD patterns show that the structure of the MWCNT support was uniformly maintained through the reaction process and that Cu and Fe3O4 NPs were also formed successfully.
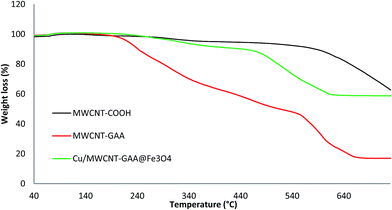
To obtain information on the thermal stability, TGA was carried out by heating MWCNT-COOH, MWCNT-GAA and Cu/MWCNT-GAA@Fe3O4 up to 700 °C under air at a heating rate of 10 °C min−1 (Fig. 4). The TGA curves showed an initial weight loss up to 200 °C because of the removal of moisture and physically adsorbed solvent. In the TGA curve of the MWCNT-COOH a second weight loss occurred between 550–700 °C, which was related to the oxidation of the purified MWCNTs. The MWCNT-GAA showed the same initial weight loss and two new stages appeared for weight loss consisting of the deformation of functional groups and decomposition of the organic linker together with MWCNT oxidation at temperatures of 220–550 °C and 550–700 °C, respectively. However, for Cu/MWCNT-GAA@Fe3O4, three temperature ranges of initial weight diminution, which were the same as the previous cases but with a difference in the amounts of weight loss, were clearly observed. The residual weights of MWCNT-COOH, MWCNT-GAA and Cu/MWCNT-GAA@Fe3O4 were 62%, 17% and 58%, respectively. Decreases in the amounts of residual weight of MWCNT-GAA compared to MWCNT-COOH were related to the tethering of GAA to MWCNT. The amounts of GAA attached to the MWCNTs can be calculated from the difference in weight loss of the MWCNT-COOH and MWCNT-GAA hybrids and was 50 wt% at 700 °C. However, the residual weight of Cu/MWCNT-GAA@Fe3O4 confirmed the addition of Cu and Fe3O4 NPs to the recently functionalized nanocomposite.
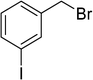
To show the efficiency of the newly synthesized nanocatalyst, the catalytic activity of Cu/MWCNT-GAA@Fe3O4 was evaluated in a click reaction for the synthesis of substituted 1,2,3-triazoles. These triazole compounds are the most important heterocycles, because of their unique chemical and structural properties and various synthetic methods have been developed for their construction.51 Recently, the applications of this building block have been extended into various research fields, such as biological science,52 material chemistry53 and medicinal chemistry.54 This investigation began with the evaluation of Cu/MWCNT-GAA@Fe3O4 as a heterogeneous catalyst in the reaction of phenylacetylene (1 mmol, 0.102 mL), benzyl bromide (1 mmol, 0.118 mL) and sodium azide (NaN3, 1 mmol, 0.065 g) as a model reaction. It was found that a yield of 9% of the cycloaddition product, 1-benzyl-4-phenyl-1H-1,2,3-triazole, was obtained for the coupling between benzyl bromide and phenylacetylene (Table 1, entry 1). Then, the versatility of the catalyst for the 1,3-dipolar cycloaddition of structurally diverse benzyl bromides and alkynes, was explored and the results are summarized in Table 1. All the substrates produced the expected cycloaddition product in H2O with very good to excellent yields.
| Entry | 1 | 2 | Triazoles | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a The reaction was carried out using 1 (1 mmol) and 2 (1 mmol) and 3 (1 mmol) in the presence of catalyst (0.020 g) at 50 °C.b Isolated yield. | |||||
| 1 | 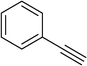 |
 |
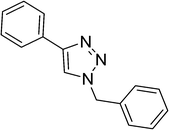 |
1 | 99 |
| 2 | 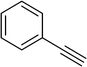 |
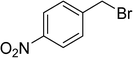 |
 |
2 | 95 |
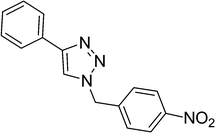
| 3 | 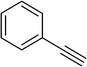 |
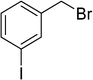 |
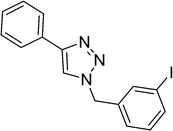 |
2 | 89 |
| 4 |  |
 |
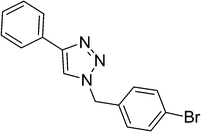 |
2 | 90 |
| 5 |  |
 |
 |
2 | 86 |
| 6 |  |
 |
 |
2 | 100 |
| 7 | 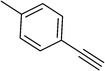 |
 |
 |
3 | 92 |
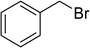
| 8 | 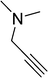 |
 |
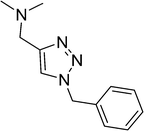 |
7 | 89 |
| 9 | 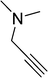 |
 |
 |
8 | 92 |
| 10 |  |
 |
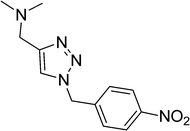 |
8 | 92 |
| 11 | 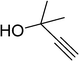 |
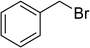 |
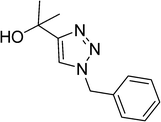 |
9 | 93 |
| 12 |  |
 |
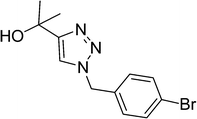 |
10 | 89 |
| 13 |  |
 |
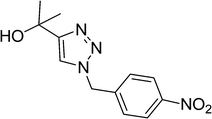 |
8 | 93 |
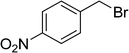
| 14 | 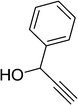 |
 |
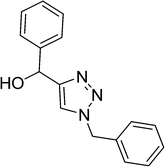 |
7 | 86 |
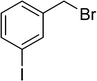
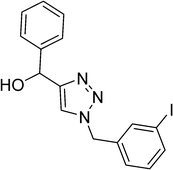
| 15 | 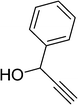 |
 |
 |
8 | 82 |
| 16 | 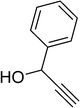 |
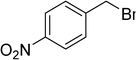 |
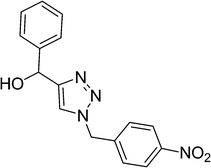 |
6 | 89 |
| 17 | 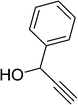 |
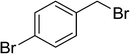 |
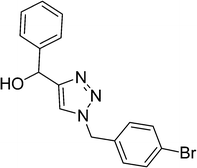 |
8 | 87 |
It is well known that H2O is considered as a green solvent for various chemical and biological reactions, and by considering both economic and environmental aspects during the course of this research, towards the development of new routes to the synthesis of organic compounds using H2O as a green medium,55–58 it was decided to used H2O as an environment friendly solvent. The results show that the click reaction was successfully carried out in H2O with good to excellent yield. As a result, there was no need for solvent optimization.
The efficiency of the synthesized catalyst in comparison with other reported catalysts is given in Table 2. The results show, in the case of Cu/MWCNT-GAA@Fe3O4, that the reaction yield is increased at the relatively shorter reaction times. The better rate of this reaction could be because of the high surface area and suitable interaction of the nanocomposite with the substrates by the electrostatic and dipole–dipole interactions. Additionally, interactions between the d-orbital of the metal and the π electron of the unsaturated linker and MWCNT could have an effect on the delocalization of the electron density over both metal and unsaturated structures of the nanocomposite. This might have an effect on the electrochemical activity of Cu NPs. Thus, these data show that the Cu NPs activity is significantly enhanced in the presence of magnetic MWCNT. Therefore, this metallic nanocomposite catalysed click reaction gives excellent yields under mild thermal conditions in H2O.
| Entry | Catalyst | Reaction conditions | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|
| a pABA: para-aminobenzoic acid, PVP: poly(vinylpyrrolidone), rt: room temperature, SiO2: silicon dioxide, t-BuOH: tert-butyl alcohol. | |||||
| 1 | PVP coated Cu–Fe3O4 NPs | t-BuOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)/rt 3)/rt |
3 | 55 | 59 |
| 2 | Cu–pABA | H2O/rt | 3 | 97 | 60 |
| 3 | Cu@Fe NPs | H2O/rt | 12 | 93 | 61 |
| 4 | SiO2–Cu | t-BuOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)/rt 3)/rt |
3 | 91 | 62 |
| 5 | Cu/MWCNT-GAA@Fe3O4 | H2O/50 °C | 1 | 99 | This work |
To optimize the required amount of catalyst, the synthesis of 1-benzyl-4-phenyl-1H-1,2,3-triazole was performed as a model reaction and the results are summarized in Table 3. Optimization of the amount of catalyst showed that 0.400 mol% of catalyst is needed to provide the best results. The optimum conditions were 0.400 mol% Cu/MWCNT-GAA@Fe3O4 and H2O as the solvent for the click reaction.
One of the advantages of this active heterogeneous nanomagnetic catalyst is its ability to perform as a recyclable reaction medium. To evaluate the level of reusability and stability of the catalyst, click experiments with phenylacetylene, benzyl bromide and NaH3 using the recycled Cu/MWCNT-GAA@Fe3O4 catalyst under the previously described conditions (Table 1, entry 1) were examined. After carrying out the first reaction, the catalyst was concentrated on the sidewall of the reaction flask using an external magnet and the catalyst was washed with acetone (2 × 5 mL) and subsequently dried at 60 °C and then reused. Only minor decreases in the reaction yield were observed after four repetitive cycles for this reaction (Fig. 5a). The decanted solution was collected for determination of Cu leaching. The Cu leaching of the catalyst was determined to be 0.002 wt% based on the FAAS analysis. Fig. 5b shows FT-IR spectra of recovered catalyst after four runs, no significant differences were observed in the peaks before and after using the nanocatalyst. Also, the TEM image proved the presence of the Cu NPs. As is shown in Fig. 5c the structure of the Cu/MWCNT-GAA@Fe3O4 nanocomposite has remained quite stable and there is no evidence of any agglomeration or defects on the MWCNT structure.
 | ||
| Fig. 5 (a) Reusability of the catalyst for the click reaction, (b) FT-IR spectra, (c) TEM image of the recovered catalyst after the fourth run. | ||
Mechanistically, it was proposed that the reaction might proceed on the surface of the Cu/MWCNT-GAA@Fe3O4 nanocatalyst by initial dipole–dipole and ionic interaction between the catalyst surface and the reagents. In the first step, benzyl azide was formed through the nucleophilic substitution reaction of the azide ion with benzyl bromide. Afterwards, the supported Cu NPs can readily insert into the terminal alkynes for polarization of the terminal triple bonds. This phenomenon was catalysed by the cycloaddition reaction of phenyl azide and alkyne to produce the target molecule (Scheme 3).
Because there is a growing demand for multi-task catalysts for a wide range of reactions and because the click reaction was carried out successfully using the prepared nanocatalyst, the usability of these catalytic systems was examined in one of the considerable condensation reactions for the synthesis of bis(indolyl)methanes was examined. Interestingly, it was found that Cu/MWCNT-GAA@Fe3O4 proved to be an efficient catalyst not only for producing 1,2,3-triazoles, but it also has a great catalytic role in the synthesis of bis(indolyl)methanes using H2O as a solvent. Results of this reaction are summarized in Table 4.
| Entry | R1 | R2 | R3 | Yielda (%) | mp (°C) (L) |
|---|---|---|---|---|---|
| a Isolated yield. | |||||
| 1 | H | H | H | 88 | 150–152 (148–149)33 |
| 2 | H | H | 4-NO2 | 96 | 215–218 (216–217)33 |
| 3 | H | H | 4-Cl | 94 | 78–79 (75–78)33 |
| 4 | H | H | 4-CH3 | 86 | 94–97 (94–95)33 |
| 5 | H | H | 2-Propargyl | 84 | 177–178 |
| 6 | Me | H | H | 92 | 188–190 (185–188)33 |
| 7 | Me | H | 2-Cl | 90 | 216–219 (220–221)33 |
| 8 | H | Br | 4-NO2 | 86 | 297–300 (294–297)33 |
The efficiency of various Cu catalysts63–66 was examined for synthesis of bis(indolyl) methanes, and the results are shown in Table 5. Among these Cu catalysts, Cu-modified MWCNT-GAA@Fe3O4 is superior, when compared to data found in the literature, for the catalyst, solvent and yield. The results showed that Cu/MWCNT-GAA@Fe3O4 nanocomposite can act as a powerful catalyst for activation of carbonyl functional groups and consequently has an interesting role in the synthesis of bis(indolyl)methanes.
A possible mechanism for the reaction between indole and benzaldehyde derivatives in the presence of Cu/MWCNT-GAA@Fe3O4 is shown in Scheme 4. It seems that the Cu/MWCNT-GAA@Fe3O4 nanocomposite activates the benzaldehyde 1 towards the electrophilic attack of indole 2 to generate indolyl carbinol, which is further converted to azafulvenium intermediate 3. The intermediate 3 can undergo further addition with a second indole molecule to produce bis(indolyl) methanes 4.
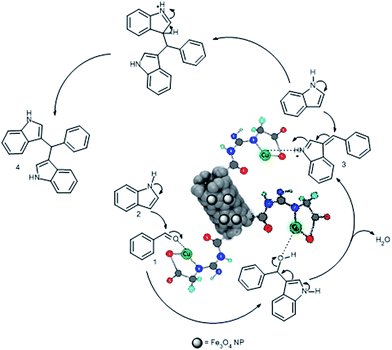 | ||
| Scheme 4 Possible reaction mechanism for the formation of bis(indolyl) methanes using the Cu/MWCNT-GAA@Fe3O4 nanocomposite. | ||
4. Conclusions
In conclusion, a Cu-modified MWCNT-GAA@Fe3O4 nanocatalyst has been developed for the first time which can act quickly and efficiently as a catalyst in organic synthesis. With this attractive and novel catalyst, a simple and efficient method has been developed for the 1,3-dipolar cycloaddition of terminal alkynes with azides and bis(indolyl)methanes in H2O as a green solvent. The advantages of using this catalyst are short times, ease of handling, high atom economy and simple work-up and all the products were also obtained in good to excellent yield and the catalyst was reusable up to four times without much loss in catalytic activity. This multi-task heterogeneous metallic-based catalyst was capable of being involved in at least two consecutive reactions having different mechanisms and represents the merger of two ubiquitous green chemistry themes: magnetic NPs being used as easily recoverable catalysts and an aqueous medium for organic reactions. Thus, these results broaden the scope of using this catalyst in the other organic chemistry reactions.4.1. Spectral data for the new derivatives of 1,2,3-triazoles
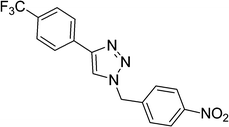
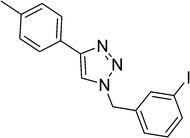
1-(4-Nitrobenzyl)-4-(4-(trifluoromethyl)phenyl)-1H-1,2,3-triazole (Table 1, entry 6). White powder; mp: 215–218 °C, 1H-NMR (300 MHz, CDCl3) δ (ppm) 5.74 (2H, s), 7.47–7.50 (2H, m), 7.68–7.71 (2H, m), 7.84 (1H, s), 7.94–7.96 (2H, m), 8.26–8.29 (2H, m); 13C-NMR (75 MHz, CDCl3) δ (ppm) 53.1, 118.1, 120.0, 120.5, 121.8, 124.9, 125.2, 127.7, 132.5, 140.1, 147.2.1-(3-Iodobenzyl)-4-p-tolyl-1H-1,2,3-triazole (Table 1, entry 7). White powder; mp 178–181 °C; 1H-NMR (300 MHz, CDCl3) δ (ppm) 2.38 (3H, s), 5.51 (2H, s), 7.09–7.14 (1H, m), 7.22–7.27 (3H, m), 7.62–7.72 (5H, m); 13C-NMR (75 MHz, CDCl3) δ (ppm) 21.3, 53.2, 94.7, 118.85, 119.25, 119.58, 125.63, 127.51, 129.50, 129.63, 130.77, 136.80, 136.97, 137.86, 138.16, 148.49, 158.53.
4.2. Spectral data for the new derivative of bis(indolyl)methane
3-((1H-Indol-3-yl)(2-(prop-2-ynyloxy)phenyl)methyl)-1H-indole (Table 2, entry 5). Dark red powder; mp 177–178 °C; 1H-NMR (300 MHz, DMSO-d6) δ (ppm) 3.58 (1H, s), 4.87 (2H, s), 6.23 (1H, s), 6.76–6.86 (4H, m), 7.13–7.36 (10H, m), 10.79 (2H, brs); 13C-NMR (75 MHz, DMSO-d6) δ (ppm) 31.9, 56.2, 78.5, 80.1, 111.8, 113.8, 118.0, 118.5, 119.4, 121.3, 123.9, 124.0, 127.1, 127.2, 129.8, 133.7, 136.8, 137.0, 154.6; analysis calculated (%) for C26H20N2O: C, 82.95; H, 5.35; N, 7.44. Found: C, 82.90; H, 5.42; N, 7.21.Acknowledgements
We gratefully acknowledge financial support from the Iran National Science Foundation (INSF), the Research Council of Shahid Beheshti University and the Catalyst Center of Excellence (CCE) at Shahid Beheshti University.References
- Y. Yan, J. Miao, Z. Yang, F.-X. Xiao, H. B. Yang, B. Liu and Y. Yang, Chem. Soc. Rev., 2015, 44, 3295–3346 RSC.
- D. R. Dreyer, H. P. Jia and C. W. Bielawski, Angew. Chem., 2010, 122, 6965–6968 CrossRef.
- J. Pyun, Angew. Chem., Int. Ed., 2011, 50, 46–48 CrossRef CAS PubMed.
- S. Liao, F. Peng, H. Yu and H. Wang, Appl. Catal., A, 2014, 478, 1–8 CrossRef CAS.
- M. B. Gawande, A. K. Rathi, I. D. Nogueira, R. S. Varma and P. S. Branco, Green Chem., 2013, 15, 1895–1899 RSC.
- I. Kalinina, Y. F. Al-Hadeethi, E. Bekyarova, C. Zhao, Q. Wang, X. Zhang, A. Al-Zahrani, F. Al-Agel, F. Al-Marzouki and R. C. Haddon, Mater. Lett., 2015, 142, 312–316 CrossRef CAS.
- Z. Liu, X. Fu, S. Tang, Y. Cheng, L. Zhu, L. Xing, J. Wang and L. Xue, Catal. Commun., 2014, 56, 1–4 CrossRef CAS.
- A. Ishibashi, Y. Yamaguchi, H. Murakami and N. Nakashima, Chem. Phys. Lett., 2006, 419, 574–577 CrossRef CAS.
- A. Satake, Y. Miyajima and Y. Kobuke, Chem. Mater., 2005, 17, 716–724 CrossRef CAS.
- L. Yang, B. Yang, D. Zeng, D. Wang, Y. Wang and L.-M. Zhang, Carbohydr. Polym., 2011, 85, 845–853 CrossRef CAS.
- R. Afshari, S. Mazinani and M. Abdouss, Nano, 2015, 10, 1550010 CrossRef CAS.
- M. B. Gawande, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 3371–3393 RSC.
- R. N. Baig and R. S. Varma, Green Chem., 2013, 15, 398–417 RSC.
- J. Andrez, G. Bozoklu, G. Nocton, J. Pécaut, R. Scopelliti, L. Dubois and M. Mazzanti, Chem.–Eur. J., 2015, 21, 15188–15200 CrossRef CAS PubMed.
- Q.-Z. Yang, A. Kermagoret, M. Agostinho, O. Siri and P. Braunstein, Organometallics, 2006, 25, 5518–5527 CrossRef CAS.
- J. L. de Miranda and J. Felcman, Polyhedron, 2003, 22, 225–233 CrossRef.
- J. Ilas, A. Mühl and S. Stöckler-Ipsiroglu, Clin. Chim. Acta, 2000, 290, 179–188 CrossRef CAS.
- M. Kuroda, Nephron, 1993, 65, 605–611 CrossRef CAS PubMed.
- J. Verhelst, J. Berwaerts, B. Marescau, R. Abs, H. Neels, C. Mahler and P. De Deyn, Metabolism, 1997, 46, 1063–1067 CrossRef CAS PubMed.
- R. Osman and H. Basch, J. Am. Chem. Soc., 1984, 106, 5710–5714 CrossRef CAS.
- J. F. Lutz, Angew. Chem., Int. Ed., 2007, 46, 1018–1025 CrossRef CAS PubMed.
- H. C. Kolb, M. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS.
- V. D. Bock, H. Hiemstra and J. H. Van Maarseveen, Eur. J. Org. Chem., 2006, 51–68 CrossRef CAS.
- S. Röper, H. C. Kolb, W. Jahnke and D. Erlanson, Click chemistry for drug discovery, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2006 Search PubMed.
- H. Nandivada, H. Y. Chen, L. Bondarenko and J. Lahann, Angew. Chem., Int. Ed., 2006, 45, 3360–3363 CrossRef CAS PubMed.
- F. Alonso, Y. Moglie, G. Radivoy and M. Yus, Org. Biomol. Chem., 2011, 9, 6385–6395 CAS.
- J. Kim, J. Park and K. Park, Chem. Commun., 2010, 46, 439–441 RSC.
- M. Bandini and A. Eichholzer, Angew. Chem., Int. Ed., 2009, 48, 9608–9644 CrossRef CAS PubMed.
- M. G. Russell, R. J. Baker, L. Barden, M. S. Beer, L. Bristow, H. B. Broughton, M. Knowles, G. McAllister, S. Patel and J. L. Castro, J. Med. Chem., 2001, 44, 3881–3895 CrossRef CAS PubMed.
- D. Maciejewska, M. Rasztawicka, I. Wolska, E. Anuszewska and B. Gruber, Eur. J. Med. Chem., 2009, 44, 4136–4147 CrossRef CAS PubMed.
- D. Kumar, V. Arun, N. Maruthi Kumar, G. Acosta, B. Noel and K. Shah, ChemMedChem, 2012, 7, 1915–1920 CrossRef CAS PubMed.
- M. Shiri, M. A. Zolfigol, H. G. Kruger and Z. Tanbakouchian, Chem. Rev., 2009, 110, 2250–2293 CrossRef PubMed.
- A. Z. Halimehjani, S. E. Hooshmand and E. V. Shamiri, RSC Adv., 2015, 5, 21772–21777 RSC.
- D. Sun, G. Jiang, Z. Xie and Z. Le, Chin. J. Chem., 2015, 33, 409–412 CrossRef CAS.
- Z. Xiang, Z. Liu, X. Chen, Q. Wu and X. Lin, Amino Acids, 2013, 45, 937–945 CrossRef CAS PubMed.
- D. Chandam, A. Mulik, P. Patil, S. Jagdale, D. Patil, S. Sankpal and M. Deshmukh, J. Mol. Liq., 2015, 207, 14–20 CrossRef CAS.
- M. Mahyari, M. S. Laeini and A. Shaabani, Chem. Commun., 2014, 50, 7855–7857 RSC.
- A. Shaabani and Z. Hezarkhani, Cellulose, 2015, 22, 3027–3046 CrossRef CAS.
- A. Shaabani, S. Keshipour, M. Hamidzad and S. Shaabani, J. Mol. Catal. A: Chem., 2014, 395, 494–499 CrossRef CAS.
- S. Keshipour and A. Shaabani, Appl. Organomet. Chem., 2014, 28, 116–119 CrossRef CAS.
- A. Shaabani and M. Mahyari, J. Mater. Chem. A, 2013, 1, 9303–9311 CAS.
- M. Mahyari and A. Shaabani, Appl. Catal., A, 2014, 469, 524–531 CrossRef CAS.
- M. Mahyari, A. Shaabani and Y. Bide, RSC Adv., 2013, 3, 22509–22517 RSC.
- A. Shaabani, M. Mahyari and F. Hajishaabanha, Res. Chem. Intermed., 2014, 40, 2799–2810 CrossRef CAS.
- M. Mahyari and A. Shaabani, J. Mater. Chem. A, 2014, 2, 16652–16659 CAS.
- Y. Liu, P. Liu, Z. Su, F. Li and F. Wen, Appl. Surf. Sci., 2008, 255, 2020–2025 CrossRef CAS.
- J. Felcman and J. L. D. Miranda, J. Braz. Chem. Soc., 1997, 8, 575–580 CrossRef CAS.
- N. Arsalani, H. Fattahi and M. Nazarpoor, eXPRESS Polym. Lett., 2010, 4, 329–338 CrossRef CAS.
- M. Salavati-Niasari and F. Davar, Mater. Lett., 2009, 63, 441–443 CrossRef CAS.
- S. Sun and H. Zeng, J. Am. Chem. Soc., 2002, 124, 8204–8205 CrossRef CAS PubMed.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS.
- M. S. Costa, N. Boechat, E. A. Rangel, F. D. C. Da Silva, A. M. De Souza, C. R. Rodrigues, H. C. Castro, I. N. Junior, M. C. S. Lourenço and S. M. Wardell, Bioorg. Med. Chem., 2006, 14, 8644–8653 CrossRef CAS PubMed.
- H. Nandivada, X. Jiang and J. Lahann, Adv. Mater., 2007, 19, 2197–2208 CrossRef CAS.
- G. C. Tron, T. Pirali, R. A. Billington, P. L. Canonico, G. Sorba and A. A. Genazzani, Med. Res. Rev., 2008, 28, 278–308 CrossRef CAS PubMed.
- A. Shaabani, M. Seyyedhamzeh, N. Ganji, M. H. Sangachin and M. Armaghan, Mol. Diversity, 2015, 19, 709–715 CrossRef CAS PubMed.
- A. Shaabani, E. Soleimani and H. R. Khavasi, Tetrahedron Lett., 2007, 48, 4743–4747 CrossRef CAS.
- A. Shaabani, A. Sarvary, S. Ghasemi, A. H. Rezayan, R. Ghadari and S. W. Ng, Green Chem., 2011, 13, 582–585 RSC.
- A. Z. Halimehjani, S. E. Hooshmand and E. V. Shamiri, Tetrahedron Lett., 2014, 55, 5454–5457 CrossRef CAS.
- N. Joshi and S. Banerjee, Tetrahedron Lett., 2015, 56, 4163–4169 CrossRef CAS.
- R. U. Islam, A. Taher, M. Choudhary, M. J. Witcomb and K. Mallick, Dalton Trans., 2015, 44, 1341–1349 RSC.
- R. Hudson, C.-J. Li and A. Moores, Green Chem., 2012, 14, 622–624 RSC.
- P. Diz, P. Pernas, A. El Maatougui, C. R. Tubio, J. Azuaje, E. Sotelo, F. Guitián, A. Gil and A. Coelho, Appl. Catal., A, 2015, 502, 86–95 CrossRef CAS.
- L. P. Mo, Z. C. Ma and Z. H. Zhang, Synth. Commun., 2005, 35, 1997–2004 CrossRef CAS.
- N. Seyedi, H. Khabazzadeh and K. Saidi, Mol. Diversity, 2009, 13, 337–342 CrossRef CAS PubMed.
- G. Meshram and V. D. Patil, Synth. Commun., 2009, 40, 29–38 CrossRef.
- A. Nareen, R. Varala and S. R. Adapa, J. Heterocycl. Chem., 2007, 44, 983 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra23294e |
| This journal is © The Royal Society of Chemistry 2016 |