Multiporous open-cell poly(vinyl formal) foams for sound absorption
Bai Xueab,
Jianguo Deng*b and
Junhua Zhang*a
aThe State Key Laboratory of Polymer Materials Engineering, Polymer Research Institute of Sichuan University, Chengdu 610065, China. E-mail: zhangjh@scu.edu.cn; Fax: +86-28-85402465
bNew Materials R & D Center, Institute of Chemical Material, China Academy of Engineering Physics, Mianyang 621900, China. E-mail: d13258430956@126.com; Tel: +86 13258430956
First published on 13th January 2016
Abstract
A series of multiporous open-cell poly(vinyl formal) (PVF) foams were obtained by crosslinking poly(vinyl alcohol) (PVA) with different contents of formaldehyde in aqueous solution. Water did not only act as the solvent of PVA, but also as the pore-forming agent during the acetalization process. With the increasing acetalization degree, the multiporous PVF foams gradually separated out from water. And the higher the acetalization degree reached, the bigger the volume shrinkage observed. PVF foams with different pore sizes were obtained by changing the formaldehyde dosage. According to the results of FTIR spectra, the intensity ratio of O–H and C–H stretching mode (νs(O–H)/νs(C–H)) could reveal the acetalization degrees of PVF foams. The thermal properties and morphologies were investigated by differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and scanning electron microscope (SEM). The PVF foams could be used as sound absorbing materials because of the open-cell multiporous structures. The sound absorption coefficient (α) of the PVF foam largely increased with the decreasing pore size, especially in the frequency range of 800–2500 Hz. And the highest α value of 0.98 was obtained at 2000 Hz for PVF-3.0.
1. Introduction
Poly(vinyl alcohol) (PVA) is one of the synthetic, water-soluble and environmentally friendly polymers, which has a number of applications in the areas of drug delivery, membranes, colloid stabilizers, adhesives, and sizing agents in textile industries.1–5 Chemical modification of PVA could be easily achieved because of its highly active hydroxyl functional groups which are available for further reactions.6 PVA crosslinked with many monomers, such as formaldehyde,7–9 glutaraldehyde,6,10 acrolein,11 fumaric acid,12 alkoxysilanes13–15 etc., usually has good chemical, thermal, and mechanical stability.Among these crosslinking reactions, PVA crosslinked with formaldehyde is identified as poly(vinyl formal) (PVF). PVF is a copolymer of unreacted vinyl alcohol and formal rings. Therefore, the properties and applications of PVF mainly depend on the relative content of functional groups of the reactants and the acetalization degree. PVF with lower acetalization degree (the molar ratio of formaldehyde to hydroxyl is less than 0.1) is still water-soluble, which can be used as adhesive.16 With the increasing formaldehyde dosage, PVF gels can be prepared and even three-dimensional solid PVF may be obtained. In order to get the three-dimensional PVF, much excessive formaldehyde is introduced to increase the acetalization degree. Flory17,18 reported that if only adjacent intramolecular hydroxyl groups participated in the acetalization, the acetalization degree was 86.46 mol% for 1,3-glycol structure and 81.6 mol% for 1,2-glycol structure.
If a suitable pore-forming agent, such as corn starch, surfactants, or the reagents19,20 that can produce gas during the reaction process (e.g. calcium carbonate21), is introduced in the acetalization process, multiporous open-cell PVF foams can be prepared. Previous researches have ever confirmed that the foams are appropriate for pressure-driven membranes designed for wastewater treatment, cells and tissue cultivation etc.22 However, the pore size of the PVF foam prepared by this method is usually larger than 25 μm.21 It seems to be difficult to obtain smaller pore size by introducing additional pore-forming agents. During the PVA acetalization process, we find that PVF foams gradually separate out from the homogeneous solution; the volumes of PVF foams gradually shrink with the increasing acetalization degree. That is to say, water is continuously excluded during the acetalization process. It gives us a hint that the solvent water may be used as the pore-forming agent to obtain multiporous structure without any other additional pore-forming agents. And it can be expected to obtain different pore sizes by changing the formaldehyde amount.
In addition, as the undesirable and hazardous noise is becoming serious and the demand for a high-class residential environment is increasing, the lightweight, thin, and low-cost substitutes of wood-based materials that are able to absorb sound in wide frequency regions have attracted a considerable research interests.23 Polymers have been widely utilized as sound-absorbing materials in the areas of buildings, machinery enclosures, duct liners, etc.24 We surprisedly find that multiporous PVF foams with open-cell structures have great potential in the application of sound-absorbent materials.
According to the train of thought, PVF foams with relatively small pore sizes (2–10 μm) were prepared by introducing different amount (the molar ratio of formaldehyde to hydroxyl groups was ranging from 0.5 to 3.0) of formaldehyde into 10 wt% PVA solution. This in-site foaming method by using water as the pore-forming agent is environmental-friendly and quite simple. The open-cell foams were further evaluated as their potential use in sound absorption. To the best of our knowledge, multiporous PVF foams using as novel sound absorbing materials are rarely reported. It is believed in this research that the sound absorption coefficient of PVF foam largely increases with the decreasing pore size.
2. Experimental
2.1 Materials
Poly(vinyl alcohol) (PVA) with a polymerization degree of ca. 1700 and a saponification degree of 99 mol% was purchased from Sichuan Vinylon Co. (China). Formaldehyde (38 wt% aqueous solution) and sulfuric acid were obtained as analytically pure reagents from Chengdu Kelong Chemical Reagent Co., Ltd (China). All the starting materials were used without further purification. Deionized water was prepared in the lab.2.2 Preparation of PVF foams
PVA (10 g) was dissolved in distilled water (90 g) at 90 °C for 6 h to obtain 10 wt% aqueous solution and then cooled to room temperature. 60 g PVA solution was added to a 250 ml three-necked round-bottomed flask with a mechanical stirrer. And a certain amount of formaldehyde and sulfuric acid (as shown in Table 1) were added into the solution with continuous agitation to make the homogeneous solution. Then the homogeneous solution was transferred to a mold and reacted at 80 °C for 5 h. The product was washed with large amount of distilled water to remove formaldehyde and sulfuric acid. After that, the product was cut into desired shape and then freeze-dried for 48 h. So a series of PVF foams containing PVF-0.5 (0.5 is the molar ratio of formaldehyde to hydroxyl groups of PVA), PVF-1.0, PVF-1.5, PVF-2.0, PVF-2.5, and PVF-3.0 were prepared.| Sample | 10% PVA (g) | 38% formaldehyde (g) | 35% sulfuric acid (g) | Formaldehyde moles per –OH |
|---|---|---|---|---|
| PVF-0.5 | 60.00 | 5.38 | 6.00 | 0.5 |
| PVF-1.0 | 60.00 | 10.77 | 6.00 | 1.0 |
| PVF-1.5 | 60.00 | 16.15 | 6.50 | 1.5 |
| PVF-2.0 | 60.00 | 21.54 | 7.00 | 2.0 |
| PVF-2.5 | 60.00 | 26.92 | 7.50 | 2.5 |
| PVF-3.0 | 60.00 | 32.31 | 8.00 | 3.0 |
2.3 Measurements
| ρ = m/v | (1) |
The morphology of PVF foam was observed by a scanning electron microscope (SEM; JEOL, JSM-5900, Japan) with an acceleration voltage of 15 kV. The samples were cryogenically fractured in liquid nitrogen. All the fractured surfaces were coated with gold to enhance the image resolution.
The equilibrium swelling of the foam was investigated by a conventional gravimetric procedure. In brief, dry foam samples were weighed on an analytical balance, and then immersed into distilled water at ambient temperature. The samples were taken out at predetermined time intervals, gently wiped with filter papers to remove excess distilled water and weighed again.
The schematic of testing sound absorption coefficient is shown in Fig. 1. A loudspeaker as a sound source was settled on the one side of the tube and the specimen was placed on the opposite side.
3. Results and discussion
3.1 FTIR analysis
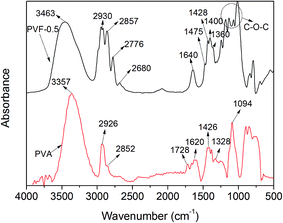
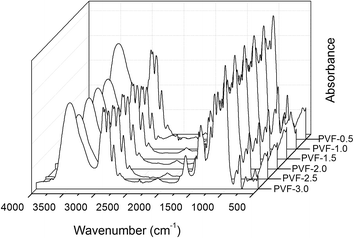
FTIR spectra of PVA and PVF-0.5 are shown in Fig. 2. In the FTIR spectrum of PVA, a broad band is observed in the range of 3640–3000 cm−1 which corresponds to the O–H stretching mode, including free hydroxyl groups and hydrogen bonds.25 However, the hydroxyl stretching band of PVF-0.5 transfers to higher wavenumber (from 3357 to 3463 cm−1) and the intensity relatively decreases, which is ascribed to the decreasing hydroxyl groups with the acetalization. The O–H bending vibration is observed at around 1640 cm−1 in both spectra of PVA and PVF. The band in the range of 2950–2900 cm−1 is considered as the asymmetric and symmetric stretching modes of –CH2 groups. In the FTIR spectrum of PVF-0.5, these bands attributed to –CH2 stretching mode split into 2930, 2857, 2776, and 2680 cm−1 because of the introduction of –O–CH2–O– with the acetalization reaction.26 And new bands also appear at 1475, 1428, 1400, 1360 cm−1 which are assigned to the C–H bending modes of PVF.26 The intensities of these PVF-0.5 bands are largely increased with the acetalization because more –CH2– groups are introduced into polymer chains. The band centered at 1094 cm−1 represents the C–O stretching vibration of PVA. The bands at 1184, 1138, 1065, and 1010 cm−1 in the FTIR spectrum of PVF-0.5 relate to C–O–C–O–C stretching vibrations.FTIR spectra of PVF foams obtained from crosslinking PVA with different contents of formaldehyde are shown in Fig. 3. It is clearly seen that the characteristic peaks of PVF as described above are all observed in the spectra of Fig. 3. It is noticed that ν(O–H) and ν(C–H) bands will change with the increasing formaldehyde amount. So the intensity ratio of O–H and C–H stretching mode (νs(O–H)/νs(C–H)) can be applied to determine the acetalization degree of PVF foam.
The influence of formaldehyde dosage on the intensity ratio (Iν(O–H)/Iν(C–H)) is illustrated in Fig. 4. It can be easily found that the value of Iν(O–H)/Iν(C–H) decreases with the increasing formaldehyde dosage. The most probable reason is that the hydroxyl groups gradually react to introduce increasing methylene groups during acetalization process. There is an obvious inflection point at the molar ratio of 1.5. When the molar ratio of formaldehyde to hydroxyl groups is less than 1.5, the Iν(O–H)/Iν(C–H) decreases quickly from 9.4 of pure PVA to 1.45; at the molar ratio greater than 1.5, the Iν(O–H)/Iν(C–H) is insensitive to the molar ratio. This demonstrates that the acetalization degree of PVF increases quickly with the incremental formaldehyde dosage at lower molar ratio of formaldehyde to hydroxyl groups. However, the acetalization degree is hardly further improved by increasing the formaldehyde dosage after the molar ratio higher than 1.5.
3.2 Foam structure characterization
The SEM images of PVF foams are shown in Fig. 5. The pore sizes and shapes of PVF foams are rather different. The pores present elliptical shapes because distortions of the foam structures could occur during the drying process.27,28The pore size and cell density (N0) of PVF foam were statistically obtained with the software Image-Pro Plus 6.0 from the SEM images. The cell density was determined using the equation as follows:
 | (2) |
The porosity of the foam was calculated as follows:31
 | (3) |
As shown in Fig. 6a, it is obviously seen that the increase of formaldehyde dosage leads to the decreasing pore size, which can be explained by an increase of the crosslinked polymer part in the volume unit. More rapid formation of the three-dimensional structure of the polymeric matrix can also cause the decrease of the pore size.32,33 The pore size decreases rapidly from 10.4 μm to 2.8 μm with the incremental formaldehyde dosage at the molar ratio of formaldehyde to hydroxyl groups less than 1.5; when the molar ratio is higher than 1.5, the pore size decreases relatively slowly from 2.8 μm to 1.9 μm. Moreover, the ratio between the major axis and minor axis also decreases with the increasing formaldehyde amount, which demonstrates that the strength of foam walls is increased and the ability of maintaining the initial shape is improved.
 | ||
| Fig. 6 (a) Influence of the molar ratio of formaldehyde to hydroxyl on the average pore size, (b) the pore size distributions of different PVF foams. | ||
Fig. 6b shows the mean diameter distributions of the PVF foams with different acetalization degree. It is clearly seen that the size distribution is narrower and the concentrated aperture size decreases with the increasing formaldehyde amount. The number of pores also rapidly increases accompanied with the reduction of pore sizes, which illustrates that the cell density increases as the formaldehyde dosage is increased.
The foam density, cell density, and porosity are summarized in Table 2. What's noticeable is that the density of PVF-0.5 (1.09 × 103 kg m−3) is higher than that of water. The foam density decreases with the incremental molar ratio of formaldehyde to hydroxyl. The strength of foam walls is improved and the ability of keeping porous structures is largely enhanced at higher formaldehyde amount. Thus the distortions of the pores during the drying process are largely decreased, which directly results in the decrease of PVF foam density. On the contrary, the porosity of PVF foam increases with the incremental formaldehyde amount.
| Sample | Foam density (kg m−3) | Cell density (cells per m3) | Porosity (%) | Equilibrium swelling degree |
|---|---|---|---|---|
| PVF-0.5 | 1090 | 1.12 × 1016 | 54.06 | 0.59 |
| PVF-1.0 | 920 | 9.06 × 1016 | 56.61 | 0.61 |
| PVF-1.5 | 500 | 2.06 × 1017 | 76.69 | 1.84 |
| PVF-2.0 | 460 | 2.96 × 1017 | 82.74 | 1.92 |
| PVF-2.5 | 410 | 7.45 × 1017 | 85.44 | 1.95 |
| PVF-3.0 | 360 | 1.84 × 1018 | 90.29 | 2.54 |
3.3 Equilibrium swelling study
The equilibrium swelling was calculated with the help of following equation:
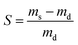 | (4) |
As shown in Fig. 5, PVF foams consist of a large number of reach-through pores occupying a considerable part of the volume unit. The reach-through pores are divided by zones of cross-linked polymer. The swelling of PVF foams is mainly due to the increase of the free-water content which does not participate in hydrogen bonds with polymer chains.34 The water absorbency directly depends on crosslinking density of the PVF foam.35
The effect of the formaldehyde dosage on the swelling of PVF foam is shown in Fig. 7. The equilibrium swelling degrees of PVF foams are listed in Table 2. The results clearly reveal that the swelling degree increases with the incremental formaldehyde dosage. It is the pore size and porosity that mainly influence the swelling degree of PVF foam. At low acetalization degree, the pore may be collapsed (as shown in Fig. 5, PVF-0.5) and the porosity decreases during the drying process. The diffusion of water into the shrunken pores is very difficult, which results in the lower equilibrium swelling degree. The strength of the pore walls increases with the incremental acetalization degree, which is beneficial to maintaining the multiporous structures and increasing the porosity. In addition, the swelling speed also increases with the increasing dosage of formaldehyde. For example, PVF-0.5 reaches the equilibrium swelling at about 110 min, however, the equilibrium swelling of PVF-3.0 only takes 10 min.
 | ||
| Fig. 7 Influence of the molar ratio of formaldehyde to hydroxyl on equilibrium swelling of the PVF foams. | ||
3.4 Thermal stability
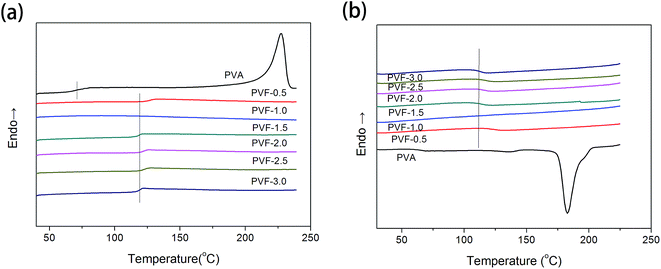
The reheating and cooling DSC curves of PVA and PVF foams are shown in Fig. 8. For clarity, all DSC scan curves shown here are shifted vertically. From Fig. 8a, it can be observed that PVA exhibits an endothermic peak at about 227 °C, corresponding to the melting temperature of PVA. No endothermic melting peaks are observed in the DSC curves of PVF foams, due to the formation of crosslinked networks between PVA and formaldehyde. The glass-transition temperature (Tg) of PVA is only 71.5 °C, however, the Tg of PVF drastically increases to around 120 °C, because crosslinked networks limit the mobility of the PVA chains. This implies that the PVF foams obtained in this study are characterized by high thermostability even heating to more than 100 °C.36–38 As shown in Fig. 8b, a single exothermic peak at about 182 °C appears in the DSC cooling curve of PVA. No exothermic peaks could be observed in the DSC cooling curves of PVF foams, which is consistent with the reheating DSC curves. The glass-transition temperatures obtained from cooling curves have no clear difference with those from reheating curves.The TGA thermograms for PVA and PVF foams are shown in Fig. 9. Not all the thermograms are shown here to gain a clear visualization. The weight loss of PVA below 150 °C is mainly due to the evaporation of free water molecules in PVA owing to its hydrophilic property.39 The weight loss of PVF foam below 150 °C is much lower than that of PVA, which is attributed to the decreasing hydrophilic property with the acetalization. As shown in TGA curves, it is clearly seen that the thermal degradation of PVF is in three steps. The initial decomposition of PVF is due to the cleavage of the formal ring and the removal of water from neighbouring pairs of unreacted hydroxyl groups.40 The threshold temperatures for weight loss of PVF-1.0, PVF-2.0, and PVF-3.0 are 192.6 °C, 181.1 °C, and 166.4 °C, and correspondingly the first peak temperatures for main weight loss are 210.9 °C, 217.4 °C, and 216.7 °C. As there are no formal rings in PVA chains, the first peak temperature for main weight loss of PVA transfers to 262.4 °C, corresponding to the removal of water from neighbouring pairs of hydroxyl groups. The second-stage degradation is due to the removal of CO, CO2, hydrocarbons, etc. from the PVF foam and the third-stage degradation of PVF is ascribed to the production of carbon.41
3.5 Acoustical property
As shown in Fig. 1, the sound pressures at two microphone locations (p1, p2) were measured and the values are shown in eqn (5) and (6):
 | (5) |
And
 | (6) |
 is the amplitude of normal incidence sound pressure and
is the amplitude of normal incidence sound pressure and  is the amplitude of normal reflection sound pressure. k0 stands for the wave number. x1 and x2 denote the distances between the two microphones and the sample, respectively.
is the amplitude of normal reflection sound pressure. k0 stands for the wave number. x1 and x2 denote the distances between the two microphones and the sample, respectively.
The sound absorption coefficient (α) could be evaluated according to the eqn (7)
| α = 1 − |γ|2 | (7) |
And
 | (8) |
Relating eqn (5)–(8), the eqn (9) could be obtained:
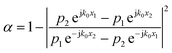 | (9) |
When the incident sound wave (Ei) transmits through the surface of the sample, it will divide into three portions: absorption sound wave (Ea), reflection sound wave (Er) and transmitting sound wave (Et), as shown in the eqn (10).
| Ei = Et + Er + Ea | (10) |
The normally incident sound absorption coefficient (α) is obtained by the eqn (11):
 | (11) |
Sound absorption coefficients of the PVF foams as the function of frequency are shown in Fig. 10a. In general, sound absorption coefficient increases with the incremental frequency, according to the Stokes–Kirchhoff formula. And the peak value of α is observed at the resonance frequency.37 For the PVF foam, the sound absorption coefficient is very low (less than 0.3) in the frequency range lower than 800 Hz. And the sound absorption coefficient fluctuates violently in this frequency range. In the higher frequency range, the sound absorption coefficient rapidly increases with the increasing frequency. The absorption peak of the sample is observed at the frequency of 2000 Hz which is ascribed to the specific characteristic of PVF foam.
 | ||
Fig. 10 (a) Sound absorption coefficients of PVF foams as a function of frequency. (b) Effect of the molar ratio of formaldehyde to hydroxyl on the average sound absorption coefficient (![[small alpha, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c2.gif) ). ). | ||
For a quantitative comparison, the average coefficients of sound absorption (![[small alpha, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c2.gif) ) for these materials can be calculated by using the following eqn (12):42
) for these materials can be calculated by using the following eqn (12):42
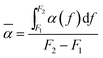 | (12) |
![[small alpha, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c2.gif) ) obviously increases with the increasing formaldehyde amount and the highest
) obviously increases with the increasing formaldehyde amount and the highest ![[small alpha, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c2.gif) value of 0.54 is obtained for PVF-3.0. The most probable reason is that the pore size decreases with the incremental acetalization degree, however, the porosity of the PVF foam increases as the dosage of formaldehyde is increased. The material with highly porous structure exhibits a good ability of normally incident sound absorption.24,43 When the incident sound wave passes through the porous material, the vibration of air in the pore and the mechanical friction between the non-rigid pore frame and air will consume large amounts of energy of the sound wave.44–47
value of 0.54 is obtained for PVF-3.0. The most probable reason is that the pore size decreases with the incremental acetalization degree, however, the porosity of the PVF foam increases as the dosage of formaldehyde is increased. The material with highly porous structure exhibits a good ability of normally incident sound absorption.24,43 When the incident sound wave passes through the porous material, the vibration of air in the pore and the mechanical friction between the non-rigid pore frame and air will consume large amounts of energy of the sound wave.44–47
4. Conclusion
In the present work, PVF foams with excellent sound absorption properties were prepared by crosslinking PVA with different formaldehyde dosage in the presence of sulfuric acid catalyst. This in-site foaming method by using water as the pore-forming agent is environmental-friendly and quite simple. The sound absorption coefficient of the PVF foam largely increases with the increasing formaldehyde dosage, especially in the frequency range of 800–2500 Hz. The highest α value of 0.98 is obtained at 2000 Hz for PVF-3.0. According to the results of FTIR analysis, the value of Iν(O–H)/Iν(C–H) decreases with the increasing formaldehyde dosage, which demonstrates that the acetalization degree of PVF is increased. However, the Iν(O–H)/Iν(C–H) decreases very slowly at the molar ratio of formaldehyde to hydroxyl groups higher than 1.5. According to SEM observations, when the molar ratio is less than 1.5, the pore size of PVF foam decreases rapidly from 10.4 μm to 2.8 μm with the increasing formaldehyde dosage; at the molar ratio higher than 1.5, the pore size decreases very slightly from 2.8 μm to 1.9 μm, which is consistent with the results of FTIR analysis. From the results of DSC, the glass-transition temperature of PVF increases to about 120 °C because the formation of networks in the acetalization process decreases the mobility of PVA chains. TGA shows the thermal degradation of PVF includes three steps: (1) the cleavage of the formal ring and the removal of water from neighbouring pairs of unreacted hydroxyl groups; (2) the removal of carbon dioxide, carbon monoxide, and hydrocarbons; (3) the production of carbon. Moreover, PVF foams with higher acetalization degrees can maintain the multiporous structures well, which is beneficial to the diffusion of water into the foams.References
- E. Chiellini, A. Corti, S. D'Antone and R. Solaro, Prog. Polym. Sci., 2003, 28, 963–1014 CrossRef CAS.
- T. Das, S. Yeasmin, S. Khatua, K. Acharya and A. Bandyopadhyay, RSC Adv., 2015, 5, 54059–54069 RSC.
- J. Li, L. Zhang, J. Gu, Y. Sun and X. Ji, RSC Adv., 2015, 5, 19859–19864 RSC.
- J. Gaume, P. Wong-Wah-Chung, A. Rivaton, S. Therias and J. L. Gardette, RSC Adv., 2011, 1, 1471–1481 RSC.
- H. Z. Zhang, B. L. Liu, R. Luo, Y. Z. Wu and D. S. Lei, Polym. Degrad. Stab., 2006, 91, 1740–1746 CrossRef CAS.
- Y. Zhang, P. C. Zhu and D. Edgren, J. Polym. Res., 2010, 17, 725–730 CrossRef CAS.
- N. Durmaz-Hilmioglu, A. E. Yildirim, A. S. Sakaoglu and S. Tulbentei, Chem. Eng. Process., 2001, 40, 263–267 CrossRef CAS.
- B. Han, J. Li, C. Chen, C. Xu and S. R. Wickramasinghe, Trans. Inst. Chem. Eng., Part A, 2003, 81, 1385–1392 CrossRef CAS.
- H. N. Chang, Desalination, 1982, 42, 63–77 CrossRef CAS.
- T. Tashima, M. Imai, Y. Kuroda, S. Yagi and T. Nakagawa, J. Org. Chem., 1991, 56, 694–697 CrossRef CAS.
- R. D. Sanderson, E. Immelman, D. Bezuidenhout, E. P. Jacobs and A. J. van Reenen, Desalination, 1993, 90, 15–29 CrossRef CAS.
- Z. Huang, H. M. Guan, W. Tan, X. Qiao and S. Kulprathipanja, J. Membr. Sci., 2006, 276, 260–271 CrossRef CAS.
- R. Guo, C. Hu, F. Pan, H. Wu and Z. Jiang, J. Membr. Sci., 2006, 281, 454–462 CrossRef CAS.
- Q. G. Zhang, Q. Liu, Z. Jiang and Y. Chen, J. Membr. Sci., 2007, 287, 237–245 CrossRef CAS.
- Y. Liu, Y. Su and J. Lai, Polymer, 2004, 45, 6831–6837 CrossRef CAS.
- S. Amiya, S. Tsuchiya, R. Qian and A. Nakajim, Pure Appl. Chem., 1990, 62, 2139–2146 CrossRef CAS.
- P. J. Flory, J. Am. Chem. Soc., 1938, 61, 1518–1521 CrossRef.
- P. J. Flory, J. Am. Chem. Soc., 1950, 72, 5052–5055 CrossRef CAS.
- N. A. Peppas and S. R. Stauffer, J. Controlled Release, 1991, 16, 305–310 CrossRef CAS.
- D. Trimnell, B. S. Shasha and F. H. Otey, J. Controlled Release, 1985, 1, 183–190 CrossRef CAS.
- X. Bai, Z. Ye, Y. Li, L. Zhou and L. Yang, Process Biochem., 2010, 45, 60–66 CrossRef CAS.
- B. Bolto, T. Tran, M. Hoang and Z. Xie, Prog. Polym. Sci., 2009, 34, 969–981 CrossRef CAS.
- S. Ersoy and H. Kucuk, Appl. Acoust., 2009, 70, 215–220 CrossRef.
- H. Zhou, B. Li and G. Huang, Mater. Lett., 2006, 60, 3451–3456 CrossRef CAS.
- B. J. Holland and J. N. Hay, Polymer, 2002, 43, 2207–2211 CrossRef CAS.
- P. Chetri and N. N. Dass, Polymer, 1997, 38, 3951–3956 CrossRef CAS.
- F. M. Plieva, M. Karlsson, M. R. Aguilar, D. Gomez, S. Mikhalovsky, I. Y. Galaev and B. Mattiasson, J. Appl. Polym. Sci., 2006, 100, 1057–1066 CrossRef CAS.
- A. A. Artyukhov, M. I. Shtilman, A. N. Kuskov, A. P. Fomina, D. E. Lisovyy, A. S. Golunova and A. M. Tsatsakis, J. Polym. Res., 2011, 18, 667–673 CrossRef CAS.
- P. Jia, J. Hu, W. Zhai, Y. Duan, J. Zhang and C. Han, Ind. Eng. Chem. Res., 2015, 54, 2476–2488 CrossRef CAS.
- D. Jahani, A. Ameli, M. Saniei, W. Ding, C. B. Park and H. E. Naguib, Macromol. Mater. Eng., 2015, 300, 48–56 CrossRef CAS.
- R. Gong, Q. Xu, Y. Chu, X. Gu, J. Ma and R. Li, RSC Adv., 2015, 5, 68003–68013 RSC.
- I. Pollers, P. Adriaensens, R. Carleer, D. Vanderzande and J. Gelan, Macromolecules, 1996, 29, 5875–5881 CrossRef CAS.
- A. A. Artyukhov, M. I. Shtilma, A. N. Kuskov, L. I. Pashkova, A. M. Tsatsakis and A. K. Rizos, J. Non-Cryst. Solids, 2011, 357, 700–706 CrossRef CAS.
- S. J. Kim, S. J. Park, K. H. An, N. G. Kim and S. I. Kim, J. Appl. Polym. Sci., 2003, 89, 24–27 CrossRef CAS.
- Y. An, T. Koyama, K. Hanabusa and H. Shirai, Polymer, 1995, 36, 2297–2301 CrossRef CAS.
- J. A. Stammen, S. Williams, D. N. Ku and R. E. Guldberg, Biomaterials, 2000, 22, 799–806 CrossRef.
- S. Eei and H. F. Nag, Polymer, 2003, 44, 1647–1653 CrossRef.
- C. M. Hassan, J. H. Ward and N. A. Peppas, Polymer, 2000, 41, 6729–6739 CrossRef CAS.
- H. Du, T. Zhou, J. Zhang and X. Liu, Anal. Bioanal. Chem., 2010, 397, 3127–3132 CrossRef CAS.
- H. Aoki and T. Suzuki, J. Polym. Sci., Part A: Polym. Chem., 1988, 26, 31–41 CrossRef CAS.
- D. A. Chatfield, J. Polym. Sci., Polym. Chem. Ed., 1983, 21, 1681–1691 CrossRef CAS.
- Y. Chen and N. Jiang, Text. Res. J., 2007, 77, 785–791 CrossRef CAS.
- X. Wang, F. You, F. S. Zhang, J. Li and S. Guo, J. Appl. Polym. Sci., 2011, 122, 1427–1433 CrossRef CAS.
- H. Zhou, B. Li, G. Huang and J. He, J. Sound Vib., 2007, 304, 400–406 CrossRef.
- H. S. Yang, D. J. Kim and H. J. Kim, Bioresour. Technol., 2003, 86, 117–121 CrossRef CAS PubMed.
- S. Ersoy and H. Kucuk, Appl. Acoust., 2009, 70, 215–220 CrossRef.
- S. Jiang, Y. Xu, H. Zhang, C. B. White and X. Yan, Appl. Acoust., 2012, 73, 243–247 CrossRef.
| This journal is © The Royal Society of Chemistry 2016 |