Polyacrylonitrile (PAN)/crown ether composite nanofibers for the selective adsorption of cations
Abstract
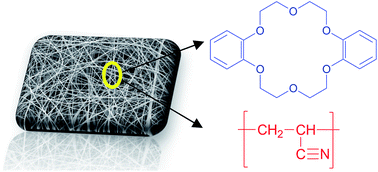
In this study, we prepared electrospun polyacrylonitrile (PAN) nanofibers functionalized with dibenzo-18-crown-6 (DB18C6) crown ether and showed the potential of these fibers for the selective recovery of K+ from other both mono- and divalent ions in aqueous solutions. Nanofibers were characterized by SEM, FTIR and TGA. SEM results showed that the crown ether addition resulted in thicker nanofibers and higher mean fiber diameters, in a range of 138 to 270 nm. Batch adsorption experiments were conducted in order to evaluate the potential of the crown ether modified nanofibers as an adsorbent for ion removal. The maximum adsorption capacity of the crown ether modified nanofibers for K+ was 0.37 mmol g−1 and the nanofibers followed the selectivity sequence of K+ > Ba2+ > Na+ ∼ Li+ for single ion experiments. Adsorption of Ba2+ ions onto crown ether-modified nanofiber was examined by XPS and the results confirmed the adsorption of the ion. Mixed ion adsorption experiments revealed competitive adsorption between K+ and Ba2+ ions for the available binding sites. This effect was not observed for the other monovalent ions present in the solution and exceptionally high selectivities for K+ over Li+ and Na+ were obtained. Also the crown ether modified nanofibers exhibited good regeneration properties and a good reusability over multiple consecutive adsorption–desorption cycles. Electrospinning is thus shown to be a very versatile tool to prepare crown ether functional polymer adsorbents for the selective recovery of ions.

 Please wait while we load your content...
Please wait while we load your content...