Fluorescence properties of dissolved organic matter as a function of hydrophobicity and molecular weight: case studies from two membrane bioreactors and an oxidation ditch†
Kang Xiaoab,
Jian-Yu Sunb,
Yue-Xiao Shenb,
Shuai Liangb,
Peng Liangb,
Xiao-Mao Wang*b and
Xia Huang*b
aCollege of Resources and Environment, University of Chinese Academy of Sciences, Beijing 100049, China
bState Key Joint Laboratory of Environment Simulation and Pollution Control, School of Environment, Tsinghua University, Beijing 100084, China. E-mail: wangxiaomao@tsinghua.edu.cn; xhuang@tsinghua.edu.cn; Tel: +86-10-62781386 Tel: +86-10-62772324
First published on 22nd February 2016
Abstract
Dissolved organic matter (DOM) plays a substantial role in wastewater treatment systems. Fluorescence is an important property of DOM and its use is promising for DOM characterization, but has rarely been extended to probing the basic physicochemical properties such as hydrophobicity and molecular weight. This study explores the possible linkages between the fluorescence properties and hydrophobicity/molecular weight of DOM, through case studies from three wastewater treatment plants (two membrane bioreactors and one oxidation ditch). The fluorescence properties of different hydrophobic/hydrophilic and molecular-weight fractions of DOM were obtained using excitation–emission matrix (EEM) spectroscopy and size-exclusion chromatography with fluorescence detection. The EEM spectra were interpreted using techniques of fluorescence regional integration, parallel factor analysis, fluorescence spectroscopic indices, and a novel energetic mapping based on fluorophore energy levels. It was found that for all the three plants, the hydrophobic fractions of DOM had a higher fluorescence intensity per UV absorbance (indicating a higher quantum yield) as well as a larger Stokes shifts than the hydrophilic fraction. The lower-molecular-weight fractions generally exhibited a higher fluorescence intensity per total organic carbon (indicating a higher fluorophore density), with the fluorescence distribution at slightly smaller excitation and emission wavelengths. These phenomena were explained via analysis of the fluorophore energy state during the excitation/emission process. The scale of the π-conjugated system in DOM molecules may serve as an intermediate factor in the correlations between the hydrophobicity/molecular weight and the fluorescence properties. These correlations may assist in developing fluorescent probes for the DOM characteristics during the process monitoring of wastewater treatment plants.
Introduction
Dissolved organic matter (DOM) plays an important role in wastewater treatment systems. DOM in the mixed liquor is inextricably linked to various processes during wastewater treatment.1,2 It can have a fundamental impact on many physicochemical processes, such as sludge sedimentation, coagulation, adsorption, and membrane filtration.3–5 Among the physicochemical properties of DOM, the hydrophobicity and molecular size/weight are important representatives.1 Hydrophobicity has a significant impact on the interfacial interactions between the DOM and surfaces/particles/colloids/solutes, and can affect the basic processes of separation, such as phase partitioning, interfacial adsorption, colloidal stability, and biopolymer solubility.6 Molecular size/weight is closely related to the steric processes of the DOM, such as diffusive mass transfer and mechanical interception (sieving effect).7 For conventional activated sludge processes like an oxidation ditch (OD), the hydrophobicity of the DOM can affect the partition equilibrium between soluble and bound extracellular polymers, which are well-reported influencers of sludge settleability;8 while the molecular weight of the DOM may impact on the mixed liquor viscosity.9 In membrane filtration processes like using a membrane bioreactor (MBR), the DOM acts as a major cause of membrane fouling which is a critical concern for filtration efficiency.4 The hydrophobicity of the DOM governs its mass balance among sludge, water, and the membrane surface,10,11 while the molecular size influences the membrane sieving and hence membrane pore blockage.7 Both the hydrophobicity and molecular size have been regarded as principal factors in membrane fouling.7,12These properties of DOM may thus serve as indicators of the treatment performance (e.g. sludge settleability and membrane retention efficiency) and the operational state (e.g. sludge activity, difficulty of separation, and propensity for membrane fouling) of the processes. Real-time continuous monitoring of the DOM characteristics, if achievable, will provide a quick understanding of the state of the wastewater treatment, and will help to optimize the process operation. For example, dynamic feedback of the DOM property data will be useful for working out preventive measures to tackle membrane fouling, by timely adjustments of the filtration conditions. Therefore, real-time DOM monitoring is expected to form a potentially critical part of the automated control and refined management of future wastewater treatment plants (WWTPs).
Despite their usefulness for monitoring wastewater treatment processes, the DOM hydrophobicity and molecular size/weight are not convenient to measure. Conventional methods (such as adsorption column chromatographic fractionation for hydrophobicity assessment,13 and size-exclusion chromatography for molecular weight profiling14) are usually complicated and time-consuming, therefore it would be difficult to achieve rapid online monitoring. Contrary in this regard, excitation–emission matrix (EEM) fluorescence spectroscopy is an attractive method, rapid and sensitive for DOM characterization.15–17 Following light absorption, the excited fluorophores of DOM undergo relaxation and emit fluorescence.18 A continuous scan of the fluorescence signals across a certain range of excitation wavelength (Ex) and emission wavelength (Em) yields a three-dimensional EEM spectrum, in which the fluorescence intensity is plotted as a function of Ex and Em. Due to its sensitive dependence upon the fluorophore properties, it has been remarked that EEM fluorescence spectroscopy is a useful tool to “fingerprint” DOM characteristics.17,19,20
The question is whether EEM fluorescence can be used to probe the properties (hydrophobicity and molecular weight) of DOM in wastewater treatment systems. Different properties of the fluorophores in terms of chemical structure, molecular polarity (related to hydrophobicity) and molecular size/weight will lead to differences in the EEM signals.21,22 Therefore, it must be theoretically feasible to use the EEM in turn to indicate differences in the hydrophobicity and molecular weight of DOM. It is speculated that for DOM in a certain unit operation of a wastewater treatment process, if there is any measurable change in the hydrophobicity or molecular weight, its fluorescence will change accordingly in a predictable way. To achieve fast monitoring of the hydrophobicity and molecular weight based on a fluorescence method, it is critical to establish the possible relationships between the fluorescence spectral information and these DOM properties.
Since DOM is a heterogeneous mixture of organic substances, a preliminary approach to examining these relationships, in principle, is to split the DOM into component mixtures with different hydrophobicity and molecular sizes, and to find out if there is any definite difference in their fluorescence. Typical components in this regard include hydrophobic acids/bases/neutrals (HOA/HOB/HON) and hydrophilic substances (HIS);1 each could be subdivided according to the molecular weight from <1 kDa to >100 kDa.23 It is conceivable that the overall fluorescence of the DOM is most likely to be of an intermediate intensity among that of the components, and vary within the limits of the components’ fluorescence. The distinction in fluorescence among these components is considered to be a fundamental condition for the relationship between the overall fluorescence and the hydrophobicity/molecular weight of the DOM. A few researchers have noted some variations of the fluorescence with variation in the DOM components according to hydrophobicity and molecular weight,5,14 however the detailed and definite relationship is yet to be systematically unveiled.
This study aims to explore the relationship between the EEM-studied fluorescence properties and the hydrophobicity/molecular weight of DOM in wastewater treatment systems. Two MBRs and an OD plant were selected as case studies, allowing cross-examination of the influence of wastewater origin and process configuration. The DOM was fractionated into different hydrophobic/hydrophilic and molecular-weight components. The information embedded in the EEM spectrum of each fraction was thoroughly explored. Combined utilization of the techniques of fluorescence regional integration (FRI),21 parallel factor analysis (PARAFAC),24 fluorescence spectroscopic indices based on ratios of the fluorescence intensity which indicate the origin of the DOM,25 and high performance size-exclusion chromatography-fluorescence detection (HPSEC-FLD)14 enabled analysis from multiple aspects. More importantly, detailed information on the fluorescence, such as fluorophore density, quantum yield,26,27 and fluorophore energetic properties (e.g. Stokes shift and energy state),18,22 was further extracted from the EEM. On this basis, we tried to find out if there are any definite differences between the DOM fractions in order to infer reasonable linkages between these fluorescence parameters and the hydrophobicity/molecular weight of the DOM.
Materials and methods
Overview of the case studies
The DOM for this study was obtained from the mixed liquor of three full-scale WWTPs: “B” and “C” are membrane bioreactors (BM and CM), and “C” is an oxidation ditch (CO) in China. BM was fed with domestic wastewater, while CM and CO were operated in parallel sharing the same municipal wastewater with an industrial portion of around 40%. All the three plants had large treatment capacities (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 d−1 for BM and 50
000 m3 d−1 for BM and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 d−1 for CM and CO), and guaranteed simultaneous removal of nitrogen and phosphorous, and the detailed process flow charts are shown in the ESI (Fig. S1†). Both BM and CM comprised anaerobic, anoxic, aerobic, and membrane tanks in sequence, while CO included an Orbal-type oxidation ditch followed by a sedimentation tank. All of the three plants were in stable operation during the period of DOM sampling.
000 m3 d−1 for CM and CO), and guaranteed simultaneous removal of nitrogen and phosphorous, and the detailed process flow charts are shown in the ESI (Fig. S1†). Both BM and CM comprised anaerobic, anoxic, aerobic, and membrane tanks in sequence, while CO included an Orbal-type oxidation ditch followed by a sedimentation tank. All of the three plants were in stable operation during the period of DOM sampling.
DOM sampling
The sludge mixed liquor was sampled from the aerobic zones of the three WWTPs (i.e., the aerobic tanks of BM and CM, and the inner channel of the Orbal oxidation ditch of CO). Considering that the aerobic zone had the longest hydraulic retention time within the process of each WWTP, samples from this zone should be the most representative of the mixed liquor of the overall process. DOM was then extracted from the mixed liquor using a sequence of: centrifugation (CF16RX II, Hitachi, Japan) at 3000 × g and 4 °C for 5 min, coarse filtration of the supernatant with filter paper, and fine filtration with a 0.7 μm glass-fiber membrane (GF/F, Whatman, UK). The resultant filtrate was regarded as the DOM solution.DOM fractionation based on hydrophobicity and molecular weight
The DOM was fractionated into HOA, HOB, HON, and HIS, according to a well-applied adsorption column chromatography procedure1,12 using a nonionic Supelite DAX-8 resin (Supelco, USA). Briefly speaking, HOA is adsorbed by the resin at lower pH but released at higher pH, while HOB behaves inversely; HON is adsorbed at any pH, while HIS is not adsorbable over the whole pH range. HOA, HOB, and HON were eluted from the resin using 0.1 M NaOH, 0.1 M HCl, and methanol, respectively. All these hydrophobic/hydrophilic fractions obtained were then diluted back to the same volume as the original DOM solution, with the pH adjusted to neutral.The hydrophobic/hydrophilic fractions of DOM were further subdivided into different molecular-size/weight grades via successive ultrafiltration,28 using a series of regenerated cellulose membranes with nominal molecular-weight cutoffs of 100 kDa, 10 kDa, and 1 kDa (PLHK, PLGC, and PLAC, Millipore, USA). The molecular-size/weight grades of the DOM fractions obtained before and after the series of ultrafiltration were thus <0.7 μm, <100 kDa, <10 kDa, and <1 kDa, respectively. The ultrafiltration was performed in a stirred dead-end filtration cell (Amicon 8400, Millipore, USA) at constant pressure, with the stirring rate set at 170 rpm to mitigate concentration polarization.
Analysis of the total organic carbon (TOC) and the concentrations of polysaccharides, proteins, and humics was employed to characterize the distribution of the DOM mass among the different hydrophobic/hydrophilic and molecular-weight fractions. The TOC was determined using a TOC analyzer (TOC-VCPH, Shimadzu, Japan). The polysaccharides were quantified according to the phenol–sulfuric acid assay.29 The proteins and humics were detected using a modified Lowry method, with interfering hard ions removed prior to the measurements.30
EEM fluorescence spectroscopy
Three-dimensional EEM spectra of the DOM fractions were obtained using a fluorescence spectrophotometer (F-7000, Hitachi, Japan). The fluorescence signals were scanned in the 3-D mode over the wavelength ranges of Ex = 200–400 nm for excitation and Em = 250–500 nm for emission, with a scan speed of 2400 nm min−1 and an interval of 5 nm. The slit widths for excitation and emission were both 5 nm, and the voltage of the photomultiplier detector was set at 700 V. Prior to the measurements, the pH of each sample was adjusted to 7.0. The EEM spectrum for each sample were measured twice and averaged. The EEM properties of the HON were not studied here, considering that the methanol contained in the HON might change the polarity of the solvent and interfere with the fluorescence measurements.31The original EEM spectra were then corrected and standardized, following the procedure illustrated in Fig. S2.† Firstly, background signals (i.e. pure water spectra) were subtracted from the EEM spectra; secondly, interfering signals of first- and second-order Rayleigh and Raman scattering were removed using an interpolation technique;32 thirdly, the fluorescence intensity was corrected for the inner-filter effect using the UV-visible absorption spectra (UV-2401PC, Shimadzu, Japan) in the range of 200–500 nm;18,33 finally, the fluorescence intensity was converted into Raman Units (R.U.) to remove instrument-dependent factors,34 and was divided by the TOC concentration to obtain the specific fluorescence intensity (S.F.I.).
The standardized EEM spectra were analyzed to harvest fluorescence information. Ordinary information obtained includes: (a) the location and intensity of the fluorescence peaks, which were directly read from the 3-D spectrum; (b) the independent fluorescent components, which were identified following the PARAFAC protocol;24 (c) the distribution of the fluorescence over different EEM regions, characterized using the FRI method;21 and (d) the fluorescence indices based on intensity ratios, including the f450/f500 index, the humification index (HIX), and the biological index of recent autochthonous contribution (BIX), which may provide clues about the origin of the DOM.25 The PARAFAC calculations were performed using MATLAB R2012b software, with 13 fluorescence samples (DOM fractions) included for each WWTP. In the FRI method, an EEM map is typically divided into five regions (denoted as I–V), which are assigned to protein-like (I and II), fulvic acid-like (III), soluble microbial byproduct-like (IV), and humic acid-like (V) substances. The fluorescence intensity in each region was integrated to calculate the percentage regional contribution to the total fluorescence. The f450/f500 index was the ratio of the fluorescence intensity at Em 450 nm to that at Em 500 nm, given that Ex = 370 nm. The HIX was obtained via dividing the integrated intensity from Em = 435–480 nm by that of Em = 300–345 nm at Ex 254 nm. The BIX was the ratio of the fluorescence intensity at Em 380 nm to that at Em 430 nm, given that Ex = 310 nm. Larger f450/f500 and BIX values, and smaller HIX values, may indicate a higher probability that the DOM has been freshly produced from biological activity.25 In-depth fluorescence information was further extracted from the EEM spectra, including: (a) the overall quantum yield, which was derived by comparing the total fluorescence intensity with the total UV absorbance;26,27 (b) the Stokes shift, calculated from the difference between the excitation and emission light frequencies;18,22 and (c) the overall energy level of the excited state, which can be obtained from the Ex and Em;18,22 and was estimated here as being negatively related to the root-mean-square (RMS) of the Ex and Em.
HPSEC-FLD
HPSEC-FLD was utilized to further inspect the dependence of the fluorescence on the molecular weight distribution for each hydrophobic/hydrophilic fraction. A fluorescence detector (FLD) was incorporated into the gel permeation/size-exclusion chromatography system (HPLC 1200 Series, Agilent Technologies, USA). The gel column (PL aquagel-OH MIXED 8 μm, Agilent Technologies, USA) allows for the separation of solutes from 0.1 to 600 kDa. For each sample, a volume of 100 μL was injected, and this was eluted with a 4 g L−1 NaCl aqueous solution at a flow rate of 0.3 mL min−1 at 30 °C. Prior to injection, the conductivity of each sample was adjusted to that of the eluent. The fluorescence signals of the effluent were recorded continuously at the Ex/Em wavelengths (nm) of 230/340, 250/430, 280/340, and 310/390, corresponding to the typical fluorescence regions for proteins, fulvic acids, microbial byproducts, and humic acids, respectively.21 In addition, the refractive index (RI) of the effluent was also monitored to reflect the overall amount of organic matter as a complement to the fluorescence signals.Results and discussion
Hydrophobicity and molecular weight distributions of the DOM
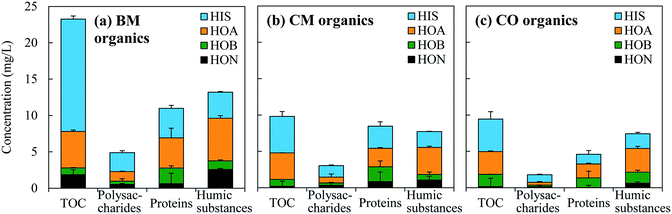
The hydrophobicity and molecular weight distributions of the DOM from the three WWTPs are shown in Fig. 1 and 2, respectively. Both the hydrophobic portion (as a sum of the HOA, HOB, and HON) and the hydrophilic portion (HIS) made significant contributions to the TOC concentration for all the three WWTPs (Fig. 1). This suggests that the fluorescence properties of both portions are worth studying.As for the chemical composition of the DOM (Fig. 1), the polysaccharides mainly behaved as HIS, and the humics were mainly enriched with HOA. The proteins contained a larger proportion than the polysaccharides and humics of HOB. The distribution of these chemical species in terms of hydrophobic/hydrophilic fractions agrees well with the nature of the typical functional groups of these species. Polysaccharides contain an abundant amount of hydroxyl groups in the sugar units,35 giving rise to a generally more pronounced hydrophilicity than for proteins and humics.11 The aromatic amines in proteins and the aromatic acids in humics are normally responsible for the hydrophobic species of the HOB and HOA, respectively.1
The hydrophobicity distributions of the DOM from the three WWTPs were not all the same (Fig. 1). The DOM from the BM plant had the highest proportion of HIS (accounting for 67% of the TOC concentration). The content of polysaccharides, proteins, and humics per unit of the TOC were generally low in the BM organics, which was particularly significant for the humics (0.57 g per g of TOC compared with 0.79 and 0.78 g per g of TOC for the CM and CO organics). These content values may indicate the functional group density. Here, the term functional groups refers to sugar units,29 peptide bonds,36 and phenolic hydroxyl groups,37 respectively, which were determined according to the specific methods for measuring polysaccharides (the phenol–sulfuric acid assay29), and proteins and humics (the modified Lowry method30). The CM and CO organics were similar in hydrophobicity distribution, except that the CM organics had a higher content of HOB and proteins (cf. Fig. 1(b) and (c)).
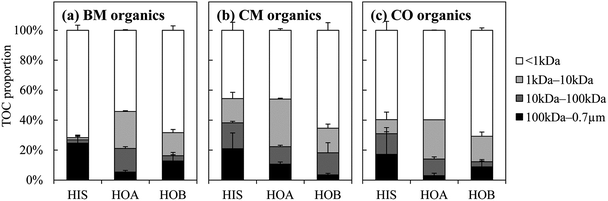
The DOM from the three WWTPs showed a broad molecular weight distribution (Fig. 2). For each of the HIS, HOA, and HOB fractions, the molecular weight distributions were similar among the three WWTPs (except for the HIS of BM). Low-molecular-weight substances (<1 kDa) formed the major portion of all of the fractions (especially for the HOB). The high- and middle-molecular-weight substances (>100 kDa and 1–100 kDa) were prominent mainly in the HIS and HOA, respectively. It was thus speculated that the average molecular weight of the hydrophobic/hydrophilic fractions might follow the order HIS > HOA > HOB. This is consistent with the general acknowledgement that polysaccharides usually have a larger average molecular size than humics in the aqueous phase of WWTPs.28,38,39
The wide distributions of the hydrophobicity and molecular weight, as shown above, could be favorable for making comparisons among the different fractions, in order to reveal the possible influence of these properties on the fluorescence.
EEM spectra of the DOM fractions
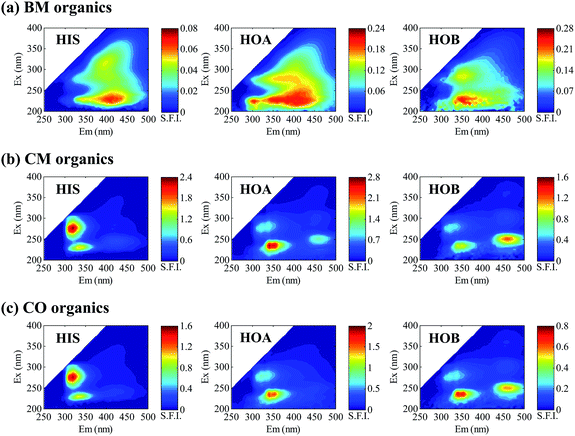
The EEM spectra of the DOM from the three WWTPs are presented in Fig. 3. Different hydrophobic/hydrophilic fractions exhibit different EEM spectra. For each fraction, the spectrum of the CM organics looks similar to that of the CO organics, but both are quite unlike that of the BM organics, which might be ascribed to the different wastewater sources of the three WWTPs. From the appearance of the EEM spectra alone, however, it is difficult to make precise comparisons of either the fractions or the WWTPs.PARAFAC analysis was performed to extract the major fluorescence components from the EEM spectra, with the components plotted in Fig. S3.† The peak locations of each component, as well as its contribution to the overall fluorescence, are given in Table S1.† The components basically covered the typical fluorescent substances of aromatic proteins, soluble microbial byproducts, humic acids, and fulvic acids. On comparison of the hydrophobic/hydrophilic fractions of the DOM (Table S1†), the fluorescence contribution rates for the PARAFAC components were different, with the hydrophobic fractions presenting a higher contribution than the hydrophilic fraction for the “A” and “B” components but a lower contribution for “D”. The difference could perhaps be somehow explained from the perspective of chemical composition. Comparison of the three WWTPs, however, was complicated and so it is difficult to draw a general conclusion.
Detailed fluorescence properties of the DOM fractions with varied hydrophobicity and molecular weights
The average fluorescence intensity per unit of TOC also varied with hydrophobicity. Among the hydrophobic/hydrophilic fractions, HOA showed the highest value for all the three WWTPs (Fig. 4(a–c)). For the BM plant, which treated domestic wastewater, the HIS exhibited the lowest AFI/TOC, likely due to the lower content of fluorescent species like proteins and humics. But for the CM and CO plants which were fed with a mixture of domestic and industrial wastewaters, the AFI/TOC levels of the HIS were largely elevated, probably because the HIS contained some highly fluorescent substances of industrial origin. The distinct EEM peaks of the HIS for the CM and CO plants (Fig. 3) may be a sign of these special fluorescent substances.
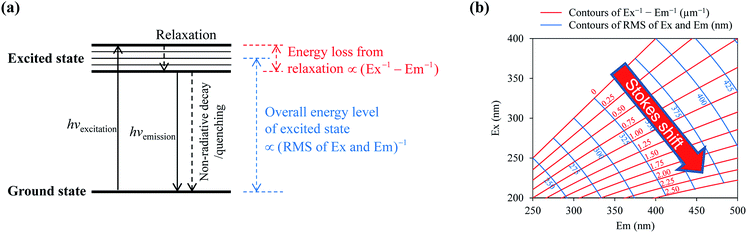 | ||
| Scheme 1 Illustration of the energy changes during a fluorescence process (a), and the contours of the fluorophore energy state on an EEM map (b). | ||
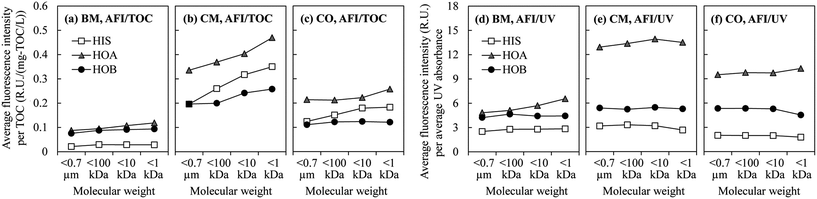
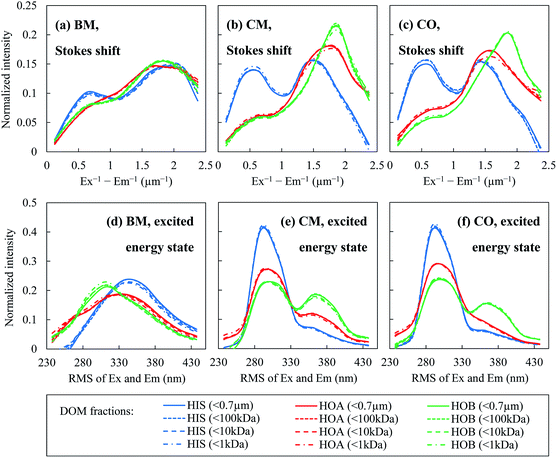
By mapping these contours onto the actual EEM spectra of the DOM fractions, one can calculate the distributions of the Stokes shift (Fig. 5(a–c)) and the RMS of the Ex and Em (Fig. 5(d–f)) in terms of the fluorescence intensity. The Stokes shift values of the hydrophilic fraction HIS were generally smaller than for the hydrophobic fractions of HOA and HOB. There were significant intensity peaks for the HIS at smaller Stokes shift values (Ex−1 − Em−1 < 1 μm−1, Fig. 5(a–c)). The hydrophobic fractions had larger Stokes shifts, i.e. a greater energy loss during vibrational relaxation, probably because they contained larger π-conjugated systems. The Stokes shift of a fluorophore can be expressed using the Lippert–Mataga equation:22
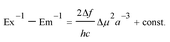 | (1) |
As for the distributions of the RMS of the Ex and Em, the peak locations and heights also varied with the hydrophobicity/hydrophilicity of the DOM fractions (Fig. 5(d–f)). However, there was no consistency among the three WWTPs as to which fraction had generally the largest or smallest RMS of the Ex and Em. On the other hand, the results from the three WWTPs seemed to agree regarding a weak dependence of the RMS upon the molecular weight. With decrease of the molecular weight, the fluorescence-intensity-weighted average excited energy state (from the average RMS calculated from the distribution curves in Fig. 5(d–f)) decreased slightly (the Spearman's rank correlation coefficients ranged from 0.8 to 1, with the exception of CO-HOB), as revealed in Table S2.† Speculatively, this trend might be related to the size of the fluorophores. It is assumed that in a pool of organic substances, the smaller molecules are more likely to bear smaller fluorophores. It is generally recognized that a smaller fluorophore leads to a greater energy gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO);22 therefore more energy is required for electronic excitation, corresponding to a higher energy level of the excited state (relative to the ground state), i.e. a smaller RMS of the Ex and Em.
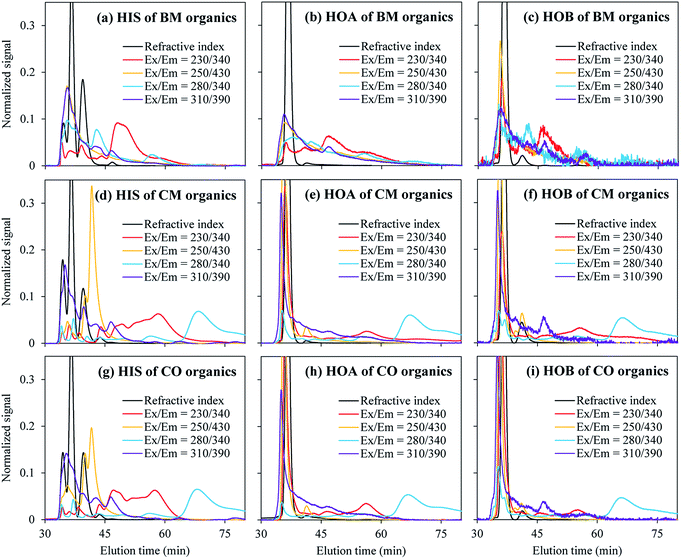 | ||
| Fig. 6 HPSEC-FLD chromatograms of the DOM fractions with different hydrophobicities from the BM, CM, and CO plants. | ||
In Fig. 6, a longer elution time indicates a smaller molecular weight. For the smaller molecular weights (e.g. an elution time larger than 45 min), stronger fluorescence signals were observed at Ex/Em = 230/340 and 280/340. These two wavelength locations have a notably smaller RMS of the Ex and Em (around 300) than those of Ex/Em = 310/390 and 250/430 (RMS around 350), corresponding to a higher energy level of the excited state. This is consistent with the finding in the previous section that the smaller DOM molecules tend to be fluorescent at a smaller RMS of the Ex and Em.
Discussion on the correlation between the fluorescence properties and hydrophobicity/molecular weight
The wastewater source of the CM and CO plants was a mixture of domestic and industrial wastewaters, while that of the BM plant was purely domestic. This could be responsible for the inter-plant differences in the DOM fluorescence properties including: the EEM appearance, PARAFAC components, FRI contribution, fluorescence indices, and overall level of the fluorophore density. Additionally, the EEM spectra of the influent wastewaters of the three WWTPs are provided in Fig. S6.†
Although sharing the same wastewater source, the CM and CO plants had different process configurations (adopting MBR and OD respectively), leading to different conditions for pollutant degradation and separation. The MBR had a longer sludge retention time (20 vs. 12 d), higher sludge concentration (∼4 vs. 2 gMLVSS L−1), and lower food-to-microorganism ratio (0.16 vs. 0.32 kgBOD5 per (kgVSS d)), which could facilitate degradation of the organics (91% ± 5% vs. 87% ± 7% for the chemical oxygen demand removal efficiency based on an annual average). The undegraded material would be partly retained by the membrane (nominal pore size of 0.1 μm) and accumulate in the mixed liquor. Thereupon, the accumulation of some less degradable fluorescent materials may provide the CM organics with a higher fluorophore density than the CO organics. The differences between the CM and CO organics in the PARAFAC components, FRI contribution, and fluorescence indices should have also originated from the different process configurations.
Despite the external effects from the wastewater source and process configuration, universal trends were found among the three WWTPs that is the seemingly internal linkages between the DOM fluorescence and hydrophobicity/molecular weight. The DOM fractions exhibited: (a) a larger Stokes shift for higher hydrophobicity, (b) a larger AFI/UV for higher hydrophobicity, and (c) a larger AFI/TOC for a smaller molecular weight. These three fluorescence parameters may have potential for probing the DOM hydrophobicity/molecular weight for future application. For example, there is a possibility that in a WWTP with online fluorescence monitoring equipment, given relatively stable external conditions (such as the wastewater source), the Stokes shift or AFI/UV may quickly reflect changes in the DOM hydrophobicity, which will be conducive to smart operation of the process. One should note that, since DOM is a complex mixture of molecules with various fluorescence characteristics, these linkages may not be accurate in every detail, but rather a macroscopic reflection of the collective properties of DOM. In order to make these potential fluorescence probing methods practicable, the “universal” trends found for the present three WWTPs will need to be confirmed through extended case studies over a range of WWTPs during their long-term operation.
Conclusions
Three WWTPs with different wastewater sources and process configurations (two MBRs and an OD) were investigated to explore the dependence of the EEM fluorescence properties on the hydrophobicity/molecular weight of the DOM. Uniform trends were found in that:(a) The hydrophobic fractions had a higher fluorescence intensity per UV absorbance (indicating a higher quantum yield) and a larger Stokes shifts than the hydrophilic fractions;
(b) The lower-molecular-weight fractions had a higher fluorescence intensity per TOC (indicating a higher fluorophore density), with the fluorescence distributed at a slightly smaller RMS of the Ex and Em.
These trends were explained from the perspective of the fluorophore energy state. A smaller RMS of the Ex and Em means a larger energy gap for electronic excitation, and a larger Stokes shift means more energy loss due to vibrational relaxation. Both are critically affected by the scale of the π-conjugated system in a fluorophore, and hence are possibly linked to the hydrophobicity and molecular weight.
An extended investigation would be required to confirm the potential commonness of these trends over a range of wastewater systems.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21407147), the special fund of the State Key Joint Laboratory of Environment Simulation and Pollution Control (no. 14K03ESPCT), and the International Program of MOST (no. 2013DFG92240).Notes and references
- J. A. Leenheer and J.-P. Croué, Environ. Sci. Technol., 2003, 37, 18A–26A CrossRef CAS PubMed.
- S. K. L. Ishii and T. H. Boyer, Environ. Sci. Technol., 2012, 46, 2006–2017 CrossRef CAS PubMed.
- R. K. Henderson, A. Baker, K. R. Murphy, A. Hambly, R. M. Stuetz and S. J. Khan, Water Res., 2009, 43, 863–881 CrossRef CAS PubMed.
- F. Meng, A. Drews, R. Mehrez, V. Iversen, M. Ernst, F. Yang, M. Jekel and M. Kraume, Environ. Sci. Technol., 2009, 43, 8821–8826 CrossRef CAS PubMed.
- S. Xue, Q. Zhao, L. Wei, X. Hui, X. Ma and Y. Lin, Front. Environ. Sci. Eng., 2012, 6, 784–796 CrossRef CAS.
- C. J. van Oss, Interfacial Forces in Aqueous Media, Taylor & Francis, Boca Raton, 2nd edn, 2006 Search PubMed.
- K. Xiao, Y.-X. Shen, S. Liang, P. Liang, X.-M. Wang and X. Huang, J. Membr. Sci., 2014, 467, 206–216 CrossRef CAS.
- S. Bala Subramanian, S. Yan, R. D. Tyagi and R. Y. Surampalli, Water Res., 2010, 44, 2253–2266 CrossRef CAS PubMed.
- M. Wloka, H. Rehage, H. C. Flemming and J. Wingender, Colloid Polym. Sci., 2004, 282, 1067–1076 CAS.
- Z. Wang, C. M. Hessler, Z. Xue and Y. Seo, Water Res., 2012, 46, 1052–1060 CrossRef CAS PubMed.
- K. Xiao, X. Wang, X. Huang, T. D. Waite and X. Wen, J. Membr. Sci., 2011, 373, 140–151 CrossRef CAS.
- Y. X. Shen, W. T. Zhao, K. Xiao and X. Huang, J. Membr. Sci., 2010, 346, 187–193 CrossRef CAS.
- G. R. Aiken, D. M. McKnight, K. A. Thorn and E. M. Thurman, Org. Geochem., 1992, 18, 567–573 CrossRef CAS.
- N. Her, G. Amy, D. McKnight, J. Sohn and Y. M. Yoon, Water Res., 2003, 37, 4295–4303 CrossRef CAS PubMed.
- C. F. Galinha, G. Carvalho, C. A. M. Portugal, G. Guglielmi, R. Oliveira, J. G. Crespo and M. A. M. Reis, Water Sci. Technol., 2011, 63, 1381–1388 CrossRef CAS PubMed.
- N. Hudson, A. Baker and D. Reynolds, River Res. Appl., 2007, 23, 631–649 CrossRef.
- Z. Wang, Z. Wu and S. Tang, Water Res., 2009, 43, 1533–1540 CrossRef CAS PubMed.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, New York, 3rd edn, 2006 Search PubMed.
- P. G. Coble, J. Lead, A. Baker, D. M. Reynolds and R. G. M. Spencer, Aquatic Organic Matter Fluorescence, Cambridge University Press, New York, 2014 Search PubMed.
- H. Yu, Y. Song, R. Liu, H. Pan, L. Xiang and F. Qian, Chemosphere, 2014, 113, 79–86 CrossRef CAS PubMed.
- W. Chen, P. Westerhoff, J. A. Leenheer and K. Booksh, Environ. Sci. Technol., 2003, 37, 5701–5710 CrossRef CAS PubMed.
- B. Valeur and M. N. Berberan-Santos, Molecular Fluorescence: Principles and Applications, Wiley-VCH, Weinheim, Germany, 2nd edn, 2012 Search PubMed.
- H.-C. Kim and B. A. Dempsey, WaterRes., 2012, 46, 3714–3722 CrossRef CAS PubMed.
- C. A. Stedmon and R. Bro, Limnol. Oceanogr.: Methods, 2008, 6, 572–579 CrossRef CAS.
- A. Huguet, L. Vacher, S. Relexans, S. Saubusse, J. M. Froidefond and E. Parlanti, Org. Geochem., 2009, 40, 706–719 CrossRef CAS.
- C. A. Parker and W. T. Rees, Analyst, 1960, 85, 587–600 RSC.
- A. T. R. Williams, S. A. Winfield and J. N. Miller, Analyst, 1983, 108, 1067–1071 RSC.
- Y.-X. Shen, K. Xiao, P. Liang, J.-Y. Sun, S.-J. Sai and X. Huang, J. Membr. Sci., 2012, 415–416, 336–345 CrossRef CAS.
- M. Dubois, K. A. Gilles, J. K. Hamilton, P. A. Rebers and F. Smith, Anal. Chem., 1956, 28, 350–356 CrossRef CAS.
- Y.-X. Shen, K. Xiao, P. Liang, Y.-W. Ma and X. Huang, Appl. Microbiol. Biotechnol., 2013, 97, 4167–4178 CrossRef CAS PubMed.
- M. Hof, V. Fidler and R. Hutterer, in Fluorescence Spectroscopy in Biology: Advanced Methods and Their Applications to Membranes, Proteins, DNA, and Cells, ed. M. Hof, R. Hutterer and V. Fidler, Springer-Verlag Berlin Heidelberg, Berlin, Heidelberg, 2005, pp. 3–29 Search PubMed.
- M. Bahram, R. Bro, C. Stedmon and A. Afkhami, J. Chemom., 2006, 20, 99–105 CrossRef CAS.
- T. Ohno, Environ. Sci. Technol., 2002, 36, 742–746 CrossRef CAS PubMed.
- A. J. Lawaetz and C. A. Stedmon, Appl. Spectrosc., 2009, 63, 936–940 CrossRef CAS PubMed.
- R. Lapasin and S. Pricl, Rheology of Industrial Polysaccharides: Theory and Applications, Blackie Academic & Professional, London, New York, 1st edn, 1995 Search PubMed.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, J. Biol. Chem., 1951, 193, 265–275 CAS.
- V. L. Singleton, R. Orthofer and R. M. Lamuela-Raventós, in Methods in Enzymology, ed. P. Lester, Academic Press, 1999, pp. 152–178 Search PubMed.
- J. Wu and X. Huang, J. Membr. Sci., 2009, 342, 88–96 CrossRef CAS.
- W.-T. Zhao, Y.-X. Shen, K. Xiao and X. Huang, Bioresour. Technol., 2010, 101, 3876–3883 CrossRef CAS PubMed.
- J. Chen, E. J. LeBoeuf, S. Dai and B. Gu, Chemosphere, 2003, 50, 639–647 CrossRef CAS PubMed.
- W. Liao, R. F. Christman, J. D. Johnson, D. S. Millington and J. R. Hass, Environ. Sci. Technol., 1982, 16, 403–410 CrossRef CAS PubMed.
- Y. Yamaguchi, Y. Matsubara, T. Ochi, T. Wakamiya and Z.-i. Yoshida, J. Am. Chem. Soc., 2008, 130, 13867–13869 CrossRef CAS PubMed.
- L. H. Fan, J. L. Harris, F. A. Roddick and N. A. Booker, Water Res., 2001, 35, 4455–4463 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra23167a |
| This journal is © The Royal Society of Chemistry 2016 |