Temperature-dependent catalytic reduction of 4-nitrophenol based on silver nanoclusters protected by a thermo-responsive copolymer ligand†
Jianhua Lüa,
Yuqin Fu*b,
Yajiao Songa,
Dongmei Wanga and
Changli Lü*a
aInstitute of Chemistry, Northeast Normal University, Changchun 130024, P. R. China. E-mail: lucl055@nenu.edu.cn
bCollege of Life Sciences, Jilin Agricultural University, Changchun 130118, P. R. China
First published on 28th January 2016
Abstract
Well-defined silver nanoclusters (NCs) were prepared by using a temperature-responsive copolymer ligand (CPL) containing 5-(2-methacryloylethyloxymethyl)-8-quinolinol (MQ) and N-isopropylacrylamide (NIPAM) units. The catalytic behaviors of the novel CPL-stabilized silver NCs were investigated through intensive kinetic evaluation using the reduction of 4-nitrophenol (4-NP) as a model reaction. The sizes of the silver NCs could be controlled in the range from 1.76 nm to 3.0 nm by the ratio of CPL and silver ion precursors. The highest catalytic activity was observed for the Ag NCs-a1. The as-prepared copolymer ligand capped Ag NCs also showed the temperature-responsive characteristics with a lower critical solution temperature (LCST) about 32 °C. The significant thermo-sensitive catalytic properties were studied in the catalytic reduction of 4-nitrophenol by the as-prepared silver nanocluster.
1. Introduction
Relative to the catalytically inert larger nanoparticles and bulk metal, noble metal nanoparticles, which can promote catalytic activity, have attracted much attention in recent years. Due to their high surface-to-volume ratio,1,2 an enormous number of active sites are available to the reactants. It is well known that silver nanoparticles have excellent catalytic activity toward many important reactions, such as the reduction of nitro-compounds,3–7 aerobic oxidation of benzylic alcohols,8 electrocatalysis for ORR,9–12 CO oxidation,13,14 selective NOx reduction15,16 and so on. Small nanoclusters have a remarkably increasing fraction of low-coordinated atoms at the surface which result in the narrow gap between the d-band and the Fermi level of low-coordinated metal atoms. Therefore, the oxygen molecules could be adsorbed on the cluster surface more easily than the close-packed counterparts, which caused much higher catalytic activity in organic molecules oxidation or oxygen reduction reactions.17For multi-functional catalysis and stabilization of nanomaterials, metal nanoparticles are both embedded in inorganic oxides such as silica, titanium dioxide and encapsulated in thermo-responsive polymeric matrix such as core–shell particles,18 core–shell microgels and micelles.19 In this way the carrier systems act not only as the stabilizer which immobilizes the particles to prevent their tendency to aggregate but also as a “nanoreactor” that leads to their response to the environment changes in temperature,20 pH,21 ionic strength22 and chemo-mechano-chemical self-regulation.23 Lu et al. demonstrated that the activity of nanoparticles could be modulated through a thermodynamic transition that takes place within the thermo-responsive core–shell particles used as a carrier that embedded Ag nanoparticles.18 Zhao et al. synthesized the bimetallic nanoparticles that exhibited both thermo- and pH-sensitivity due to the particles protected by a double stimuli-sensitive diblock copolymers.24 Up to now, the loading metal nanoparticles onto responsive hydrogels, microgels and micelles have been achieved mostly. The catalytic processes can be modulated by changing the conformation of polymer chains to expose or hide functional groups or metallic nanoparticles in the polymer matrices. To prepare thermo-responsive nanoparticles with a lower critical solution temperature (LCST) in water, many monomers have been used, such as N-isopropylacrylamide (NIPAM) and N-vinyl caprolactam (VCL).25–27 Yan et al. also synthesized thermally responsive gold nanocatalysts based on a modified polyvinylpyrrolidone.25,26
In this paper, we fabricated the novel silver nanoclusters (NCs) protected with a novel copolymer ligand (CPL) containing 8-hydroxyquinoline (HQ) and thermo-responsive poly(isopropylacrylamide) (pNIPAM) segments. The CPL macromolecules can coordinate to the surface of silver particles to form stable complexes, and the silver particles with different sizes smaller than 3 nm can be obtained by controlling the ratio of CPL and Ag ion precursors. The temperature-dependent catalytic properties for the reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) by NaBH4 using CPL-Ag NCs as catalyst were detailed investigated in this article. The catalytic activities of the Ag NCs can be modulated by the temperature over a given range.
2. Experimental
2.1 Materials
Silver nitrate (AgNO3, 99%) was obtained from Sinopharm Chemical Reagent Co. Ltd. L-Ascorbic acid (L-Aa) was purchased from Beijing chemical works. 8-Hydroxyquinoline (HQ) was purchased from Xiya Reagent. N-Isopropylacrylamide (NIPAM, Aldrich) was purchased from Tokyo Kasei Kogyo Co. Ltd. and recrystallized by hexane. NaBH4 was obtained from Sinopharm Chemical Reagent Co. Ltd. p-Nitrophenol (4-NP) was purchased from Tianjin Guangfu fine chemical research institute. Other chemicals were of analytical grade without purification. All the water we used is deionized water.2.2 Synthesis of copolymer ligand of p(NIPAM-co-MQ)
5-(2-Methacryloylethyloxymethyl)-8-quinolinol (MQ) was synthesized according to a previous procedure.28 The copolymer ligand (CPL) of p(NIPAM-co-MQ) was prepared by conventional free radical copolymerization of NIPAM and MQ monomers as described in our previous work (see Fig. S1, ESI†).29 Briefly, to a three-neck round bottomed flask 2 g NIPAM, 0.1 g MQ, 0.06 g AIBN and 40 mL of THF solution were added. The mixture was stirred and degassed under nitrogen purge before heated gradually to 60 °C. After the polymerization for 10 h, the resulting products were reprecipitated by petroleum ether and washed several times. Finally, the obtained CPL was dried under vacuum for one day at room temperature. 1H NMR data revealed that the molar ratio of NIPAM to MQ units in CPL was 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The number average molecular weight (
1. The number average molecular weight (![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n) of the copolymer was determined by GPC to be around 26
n) of the copolymer was determined by GPC to be around 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 with a polydispersity index (PDI) of 1.63. 1H NMR (500 MHz, CDCl3, see Fig. S2, ESI†): 8.78 ppm (1H, Ph-H), 8.50 ppm (1H, Ph-H), 7.46 ppm (1H, Ph-H), 7.40 ppm (1H, Ph-H), 7.08 ppm (1H, Ph-H), 6.03 ppm (1H,
000 with a polydispersity index (PDI) of 1.63. 1H NMR (500 MHz, CDCl3, see Fig. S2, ESI†): 8.78 ppm (1H, Ph-H), 8.50 ppm (1H, Ph-H), 7.46 ppm (1H, Ph-H), 7.40 ppm (1H, Ph-H), 7.08 ppm (1H, Ph-H), 6.03 ppm (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.52 ppm (1H,
CH2), 5.52 ppm (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.87 ppm (2H, OCH2OPh), 4.27–3.69 ppm (4H, OCH2CH2OO), 1.88 ppm (3H, OCH3).
CH2), 4.87 ppm (2H, OCH2OPh), 4.27–3.69 ppm (4H, OCH2CH2OO), 1.88 ppm (3H, OCH3).
2.3 Preparation of Ag NCs
108, 545 and 1090 μL (5 mg mL−1) CPL Milli-Q aqueous solution were added into the right amount of water to prepare 4.8 mL CPL solution. 0.1 mL AgNO3 solution (1 mg mL−1) was added into the CPL Milli-Q aqueous solution with different concentration (the molar feed ratios of Ag/CPL is denoted as [AgNO3]/[MQ units] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, 1
0.1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and 1
0.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) under vigorous stirring. After two hours, 81 μL L-ascorbic acid solutions (5 mg mL−1) were added under vigorous stirring. The pH values of the solutions were adjusted to 10 with ammonia aqueous solution. The final volume of the reaction solution is 5 mL. The solution was incubated at room temperature for 20 h, then the final obtained CPL capped Ag nanoclusters (CPL-Ag NCs) were dialyzed three days. Finally, the as-prepared solutions were stored at 4 °C before use.
1) under vigorous stirring. After two hours, 81 μL L-ascorbic acid solutions (5 mg mL−1) were added under vigorous stirring. The pH values of the solutions were adjusted to 10 with ammonia aqueous solution. The final volume of the reaction solution is 5 mL. The solution was incubated at room temperature for 20 h, then the final obtained CPL capped Ag nanoclusters (CPL-Ag NCs) were dialyzed three days. Finally, the as-prepared solutions were stored at 4 °C before use.
2.4 Catalytic reduction of 4-nitrophenol
The catalytic reduction reaction was conducted in a standard quartz cuvette with a 1 cm path length. For the temperature responsive catalytic experiments, an aliquot solution of CPL-Ag NCs was heated to a given temperature, and then was added to the preheated aqueous solution contains 1.5 mL of fresh NaBH4 aqueous solution (11.4 mg NaBH4) and 1.5 mL of 4-NP aqueous solution (0.2 mM). The final concentrations of 4-NP and NaBH4 for all of the multivariate experiments were 0.088 and 88 mmol L−1. The absorption spectra were monitored in the range of 250–550 nm at different temperatures from 24 to 42 °C.2.5 Measurements
1H NMR spectra were obtained from an AVANCE Bruker spectrometer at basic frequencies of 500 MHz for 1H in CDCl3 solution. UV-vis absorption spectra were recorded on a SHIMADZU UV-2550 UV-visible spectrophotometer in the range 200–800 nm. The molecular weight of polymer was estimated at a flow rate of 1.0 mL min−1 at 25 °C by gel permeation chromatography (GPC) equipped with Waters 1515 pump and Waters 2414 differential refractive index detector. CHCl3 was used as eluent, and the molecular weight was determined vs. polystyrene standards. Transmission electron microscopy (TEM) was carried out on a JEM-2100F electron microscope.3. Results and discussion
3.1 Characterization of CPL-Ag NCs
Ag NCs were prepared by using the temperature-responsive copolymer ligand (CPL) as stabilizer which was obtained via the copolymerization of monomers of 5-(2-methacryloylethyloxymethyl)-8-quinolinol (MQ) and N-isopropylacrylamide (NIPAM) (Scheme 1). The synthesized samples from a1 to a3 represent the CPL-capped Ag NCs (CPL-Ag NCs) with the molar feed ratios of [AgNO3]/[MQ units] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 (a1), 1
0.1 (a1), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 (a2) and 1
0.5 (a2) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (a3). It is well known that the large silver nanoparticles usually have an absorption band between 400 and 500 nm,30 whereas small nanoclusters exhibit different absorption profiles due to that the scarce electrons cannot support a surface plasmon resonance.31 Fig. 1 shows the UV-visible absorption spectra of CPL and different CPL-Ag NCs. It can be observed that the different CPL-Ag NCs have the absorption peak at 260 nm assigned to the p–p* electron transition from the quinoline ring (electron donor) of MQ units in CPL, indicating that CPL has coordinated on the surface of Ag NCs.23 In addition, there is no characteristic surface plasmon resonance absorption peak around 400–500 nm for different CPL-Ag NCs, which illustrates that no larger Ag nanoparticles are formed in our systems.32,33
1 (a3). It is well known that the large silver nanoparticles usually have an absorption band between 400 and 500 nm,30 whereas small nanoclusters exhibit different absorption profiles due to that the scarce electrons cannot support a surface plasmon resonance.31 Fig. 1 shows the UV-visible absorption spectra of CPL and different CPL-Ag NCs. It can be observed that the different CPL-Ag NCs have the absorption peak at 260 nm assigned to the p–p* electron transition from the quinoline ring (electron donor) of MQ units in CPL, indicating that CPL has coordinated on the surface of Ag NCs.23 In addition, there is no characteristic surface plasmon resonance absorption peak around 400–500 nm for different CPL-Ag NCs, which illustrates that no larger Ag nanoparticles are formed in our systems.32,33
 | ||
| Scheme 1 Schematic representation of synthesis and responsive catalysis of thermo-responsive CPL-Ag NCs. | ||
 | ||
| Fig. 1 UV-vis absorption spectra of CPL and the as-prepared CPL-AgNCs-a1, a2, a3 and their digital images, respectively. | ||
TEM characterization was used to further confirm the formation of CPL-Ag NCs. Fig. 2 shows the TEM images and size distribution histograms of CPL-Ag nanocluster samples a1 to a3 with different Ag/CPL ratios. It can be seen that the CPL-Ag NCs have an average size of 3.01 ± 1.1 nm (a1), 2.38 ± 0.51 nm (a2) and 1.76 ± 0.36 nm (a3). HRTEM of sample a2 shows the characteristic 0.24 nm spacing ascribed to the crystal lattice spacing of Ag (111). So, it can be demonstrated that the CPL-Ag NCs have been synthesized successfully and the sizes of the resulting Ag NCs can be effectively modulated by the ratio of copolymer ligand (CPL) and Ag ion precursors. Interestingly, it can be noted the CPL-Ag NCs a2 and a3 also exhibit the self-assembled chain structures (Fig. S3, ESI†), indicating that the temperature-responsive copolymer ligand can induce the self-assembly of the formed Ag NCs along the polymer chains via the coordination effect of 8-hydroxyquinoline ligand on CPL side chains.
 | ||
| Fig. 2 TEM images and size distribution histograms of CPL-Ag NCs-a1 (A), a2 (B), a3 (C) and (D) size change of CPL-Ag NCs with decreasing Ag/CPL molar ratios. | ||
Fig. 3 shows the temperature dependence of light transmittance of the aqueous solutions of the thermo-responsive copolymer ligand (CPL) and CPL-capped Ag NCs a1–a3. Both the copolymer ligand and CPL-Ag NCs-a3 in water showed a lower critical solution temperature (LCST) at about 32 °C as a result of the conformational change of PNIPAM chains. The CPL-Ag NCs could disperse stably in water when the temperature was below 32 °C. Followed by the raise of temperature to above 32 °C, it could be observed that the transparent solution remarkably became turbid due to the phase transition of thermo-responsive CPL networks.23,29 Furthermore, once the turbid solution was cooled to ambient temperature, the turbid suspension redispersed in water to become a transparent solution. We also found that the temperature-responsive behavior of CPL-Ag NCs-a3 was highly reversible. However, the as-prepared CPL-Ag NCs-a1 and CPL-Ag NCs-a2 did not possess obvious volume transition due to the very low amount of surface capping copolymer ligand.
 | ||
| Fig. 3 Temperature dependence of light transmittance of aqueous solutions of CPL and CPL-Ag NCs-a1, a2 and a3 at 600 nm. | ||
3.2 Responsive catalytic performance of CPL-Ag NCs in 4-NP reduction
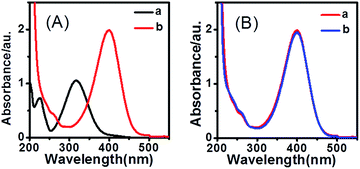
The catalytic properties of the stable Ag NCs were examined for the reduction of 4-nitrophenol by NaBH4, which is one of the model reactions for evaluating the catalytic activity of noble nanoparticles. As can be seen from the absorption spectra (see Fig. 4A) of 4-NP in absence and presence of NaBH4, the characteristic absorption peak at 317 nm ascribed to 4-nitrophenol solution is obviously red-shifted to 400 nm when 4-NP is treated with an aqueous solution of NaBH4. At the same time, the color of the solution changed from light yellow to bright yellow-green due to the formation of 4-nitrophenolate ions. The characteristic band of 400 nm for the mixture solution of 4-NP and NaBH4 decreased slightly in the intensity after 24 h, suggesting that the reduction did not proceed in absence of a catalyst (see Fig. 4B). This is because the reaction of 4-NP to 4-aminophenol (4-AP) by only NaBH4 is a thermodynamically feasible but kinetically restricted process in the absence of a catalyst.34 The UV-vis spectra for the reduction of 4-NP by NaBH4 using different CPL-Ag NCs are shown in Fig. 5. The maximum absorption peak centered at 400 nm gradually diminished with the addition of 300 μL CPL-Ag NCs to the reaction mixture solution, and the temporal evolution kinetics could be monitored by UV-vis spectroscopy. In the meantime a new peak at around 300 nm appeared as a result of the formation of 4-AP. The color of the solution faded from bright yellow-green to colorless due to the conversion from 4-NP to 4-AP (see Fig. S4, ESI†).The concentration of NaBH4 far exceeds to the concentration of 4-NP, thus the reduction rate kinetics can be considered as pseudo-first-order kinetics, where the kinetic rate r is often defined (eqn (1)).35,36
 | (1) |
 | (2) |
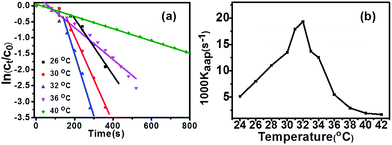
We have calculated the reaction rate constants by measuring the absorbance of 4-NP at 400 nm with the reaction time to compare the catalytic activities of CPL-Ag NCs with a1–a3 (Fig. 5a–c). The reaction maintains the pseudo-first order kinetics, and a linear relationship between ln(ct/c0) and reaction time (t) is obtained for all the catalytic procedure (see Fig. 5d). The calculated rate constants for the different catalysts of CPL-Ag NCs (a1, a2, a3) are 0.91, 0.11 and 0.05 min−1, respectively. It is well known that the 4-NP can be reduced by NaBH4 to 4-AP through transferring the electrons from BH4− to 4-NP via the CPL-Ag NCs.37,38 In our work, the efficiency of catalytic reaction decreases from CPL-Ag NCs a1, a2 to a3 with the deceasing molar feed ratios of Ag/CPL. The possible reason is that the large number of copolymers enwrapped the Ag NCs in CPL-Ag NCs a2 to a3 via the strong coordination interaction of 8-hydroxyquinoline segments, which prevented the reactants from contacting with the catalyst of Ag NCs.39
Because the CPL has a sensitive thermally triggered response, the aggregation of CPL-Ag NCs-a1 networks upon heating may lead to the decline of catalytic activity due to the volume transition of CPL. Although an obvious phase transition of spherical CPL-Ag NCs-a1 was not found, its responsible catalytic activities with the temperature were studied due to its high catalytic reduction activity. To characterize the responsive catalysis of thermo-responsive CPL-Ag NCs-a1, the reduction of 4-NP by excess NaBH4 with CPL-Ag NCs-a1 as a catalyst at different temperatures was selected as a model reaction. A linear relationship between ln(ct/c0) and the reaction time t was obtained with the range from 24 °C to 42 °C (Fig. 6a). Fig. 6b shows the dependence of rate constant kapp of the reduction reaction catalyzed with the thermo-responsive CPL-Ag NCs-a1 on the temperature in the range from 24 to 32 °C. Instead of a common linear relationship between kapp and T according to the representation of Arrhenius equation, the changes of kapp with temperature are intriguing in our study. It is generally recognized that there are two mainly factors that affect the apparent rate constant kapp with temperature in the composite hydrogel and microgels.40 On the one hand, the rate constant kapp of the catalytic reaction increases as the temperature increases, which is consistent with the representation of Arrhenius equation. On the other hand, the diffusion speed of reactants to the Ag particles in the network also determines the reaction rate. In this study, the kapp initially raised with the increase of temperature below 32 °C, which followed the typical Arrhenius-type dependence on temperature. At this range, the barrier of the CPL chains is so weak that the reactants can easily penetrate the barrier of CPL and quickly contact with Ag NCs, as a result, the catalytic rate increased. However, the catalytic activities exhibited dramatically inverse relationship between kapp and T at the range from 32 °C to 42 °C. The possible reason was that the shrinkage of the temperature-responsive pNIPAM segments slow the diffusion rate of reactants towards Ag NCs, thus decreasing the rate constant.35,41 With the temperature above 32 °C, the pNIPAM segments change from hydrophilic to hydrophobic and shrink on the silver nanoparticles to form a barrier which prevent the reactants from contacting with the catalyst of Ag NCs.35,41 This demonstrates that the mode of tuning the shrinkage and stretch of a thermosensitive carrier hold great promise in intelligent controlling catalytic activity.
4. Conclusions
In summary, the thermosensitive silver nanoclusters as catalysts were developed using p(NIPAM-co-MQ) as ligands in aqueous solution, and the size of the formed Ag NCs could be modulated by the ratio between the CPL and Ag precursor ions. It had been demonstrated that the CPL-Ag NCs-a1 exhibited excellent thermo-responsible catalytic properties for the reduction reaction of 4-nitrophenol. The catalytic activity of CPL-Ag NCs could be effectively modulated by the volume transition of the copolymer ligand on the surface of Ag NCs. So, the CPL not only can combine with Ag+ to form Ag(0) but also can possess thermo-responsive function to modulate the catalytic activities of Ag NCs. The present proposed approach could potentially create new opportunity for the synthesis of similar multi-responsible metal nanohybrid catalyst.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21574017).Notes and references
- D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852 CrossRef CAS PubMed.
- X. Zhou, W. Xu, G. Liu, D. Panda and P. Chen, J. Am. Chem. Soc., 2010, 132, 138 CrossRef CAS PubMed.
- W. C. Elias, R. Eising, T. R. Silva, B. L. Albuquerque, E. Martendal, L. Meier and J. B. Domingos, J. Phys. Chem. C, 2014, 118, 12962 CAS.
- T. Premkumar and K. E. Geckeler, Mater. Chem. Phys., 2014, 148, 772 CrossRef CAS.
- S. Wu, S. Yan, W. Qi, R. Huang, J. Cui, R. Su and Z. He, Nanoscale Res. Lett., 2015, 10, 213 CrossRef PubMed.
- M. Li and G. Chen, Nanoscale, 2013, 5, 11919 RSC.
- M. Russo, F. Armetta, S. Riela, D. Chillura Martino, P. L. Meo and R. Noto, J. Mol. Catal. A: Chem., 2015, 408, 250 CrossRef.
- S. Panigrahi, S. Basu, S. Praharaj, S. Pande, S. Jana, A. Pal, S. K. Ghosh and T. Pal, J. Phys. Chem. C, 2007, 111, 4596 CAS.
- M. Liu and W. Chen, Nanoscale, 2013, 5, 12558 RSC.
- S. Jin, M. Chen, H. Dong, B. He, H. Lu, L. Su, W. Dai, Q. Zhang and X. Zhang, J. Power Sources, 2015, 274, 1173 CrossRef CAS.
- C. Jeyabharathi, S. S. Kumar, G. V. Kiruthika and K. L. Phani, Angew. Chem., Int. Ed., 2010, 49, 2925 CrossRef CAS PubMed.
- M. Liu and W. Chen, Nanoscale, 2013, 5, 12558 RSC.
- D. Tang, Z. Chen, J. Hu, G. Sun, S. Lu and C. Hu, Phys. Chem. Chem. Phys., 2012, 14, 12829 RSC.
- S. Chen, L. Luo, Z. Jiang and W. Huang, ACS Catal., 2015, 5, 1653 CrossRef CAS.
- K. Shimizu, K. Sawabe and A. Satsuma, Catal. Sci. Technol., 2011, 1, 331 CAS.
- K. Paredis, L. K. Ono, F. Behafarid, Z. Zhang, J. C. Yang, A. I. Frenkel and B. R. Cuenya, J. Am. Chem. Soc., 2011, 133, 13455 CrossRef CAS PubMed.
- Y. Lu and W. Chen, J. Power Sources, 2012, 197, 107 CrossRef CAS.
- Y. Lu, Y. Mei, M. Drechsler and M. Ballauff, Angew. Chem., Int. Ed., 2006, 45, 813 CrossRef CAS PubMed.
- L. Li, H. Qian and J. Ren, Chem. Commun., 2005, 4, 528 RSC.
- B. Liu, Y. Wang, M. Deng, J. Lü, C. Tong and C. Lü, RSC Adv., 2014, 4, 57245 RSC.
- X. Chen, Y. An, D. Zhao, Z. He, Y. Zhang, J. Cheng and L. Shi, Langmuir, 2008, 24, 8198 CrossRef CAS PubMed.
- G. Li, C. A. Tai, K. G. Neoh, E. T. Kang and X. Yang, Polym. Chem., 2011, 2, 1368 RSC.
- X. He, M. Aizenberg, O. Kuksenok, L. D. Zarzar, A. Shastri, A. C. Balazs and J. Aizenberg, Nature, 2012, 48, 7214 Search PubMed.
- D. Zhao, X. Chen, Y. Liu, C. Wu, R. Ma, Y. An and L. Shi, J. Colloid Interface Sci., 2009, 331, 104 CrossRef CAS PubMed.
- N. Yan, J. Zhang, Y. Yuan, G. Chen, P. J. Dyson, Z. Li and Y. Kou, Chem. Commun., 2010, 46, 1631 RSC.
- M. Beija, J. D. Marty and M. Destarac, Chem. Commun., 2011, 47, 2826 RSC.
- J. Zhang, Y. Yuan, K. J. Kilpin, Y. Kou, P. J. Dyson and N. Yan, J. Mol. Catal. A: Chem., 2013, 371, 29 CrossRef CAS.
- N. Du, R. Tian, J. Peng and M. Lu, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 397 CrossRef CAS.
- B. Liu, C. Tong, L. Feng, C. Wang, Y. He and C. Lü, Macromol. Rapid Commun., 2014, 35, 77 CrossRef CAS PubMed.
- J. J. Mock, M. Barbic, D. R. Smith, D. A. Schultz and S. Schultz, J. Chem. Phys., 2002, 116, 6755 CrossRef CAS.
- L. Zhang and E. Wang, Nano Today, 2014, 9, 132 CrossRef CAS.
- Z. Yuan, N. Cai, Y. Du, Y. He and E. S. Yeung, Anal. Chem., 2014, 86, 419 CrossRef CAS PubMed.
- J. Lan, P. Zhang, T. Wang, Y. Chang, S. Lie, Z. Wu, Z. Liu, Y. Li and C. Huang, Analyst, 2014, 139, 3441 RSC.
- J. Liu, X. Ren, X. Meng, Z. Fang and F. Tang, Nanoscale, 2013, 5, 10022 RSC.
- G. Liu, D. Wang, F. Zhou and W. Liu, Small, 2015, 11, 2807 CrossRef CAS PubMed.
- (a) S. Kundu, Phys. Chem. Chem. Phys., 2013, 15, 14107 RSC; (b) X. Liu, F. Cheng, Y. Liu, H. Liu and Y. Chen, J. Mater. Chem., 2010, 20, 360 RSC.
- L. B. Devi and A. B. Mandal, RSC Adv., 2013, 3, 5238 RSC.
- H. Zhang, X. Li and G. Chen, J. Mater. Chem., 2009, 19, 8223 RSC.
- Y. Liu, L. Liu, M. Yuan and R. Guo, Colloids Surf., A, 2013, 417, 18 CrossRef CAS.
- C. Zhu, Z. Hai, C. Cui, H. Li, J. Chen and S. Yu, Small, 2012, 8, 930 CrossRef CAS PubMed.
- Y. Wang, G. Wei, F. Wen, X. Zhang, W. Zhang and L. Shi, J. Mol. Catal. A: Chem., 2008, 280, 1 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra23158b |
| This journal is © The Royal Society of Chemistry 2016 |