Catalytic activity of bare and porous palladium nanostructures in the reduction of 4-nitrophenol†
Abstract
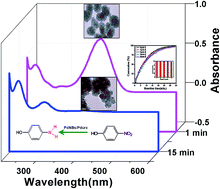
The catalytic activity of bare and porous palladium nanostructures viz. palladium nanoballs (PdNBs) and palladium urchins (Pdurc) synthesized in surfactant based liquid crystalline mesophase have been investigated in the reduction of 4-nitrophenol. The loading of PdNBs and Pdurc nanocatalyst in the reaction are optimized to 1.57 × 10−4 mol% and 1.57 × 10−3 mol% respectively. The surface area normalized rate constant obtained at 303 K for PdNBs (0.74 ± 0.03) s−1 m−2 L and Pdurc (0.41 ± 0.05) s−1 m−2 L is the highest, considering the low palladium loading used in the reaction. The specific surface area determined for the PdNBs and Pdurc are 153 m2 g−1 and 27 m2 g−1 respectively. The high surface area of PdNBs nanostructure is consistent with the highest catalytic activity. The palladium nanostructure catalyzed reaction lacks induction time (t0), indicating the influence of porosity in the Langmuir–Hinshelwood mechanism. Besides, PdNBs and Pdurc exhibited thermal stability and structural integrity with good conversion of the substrate (>97%) even up to five consecutive reactions. The mechanistic insight of the bare and porous palladium nanostructures presented in this study is significant for the development of stable and efficient palladium metal based nanocatalyst.

 Please wait while we load your content...
Please wait while we load your content...