Synthesis, structure and characterization of a layered coordination polymer based on Zn(ii) and 6-(methylmercapto)purine†
Abstract
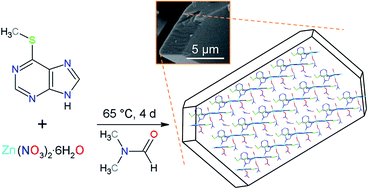
A layered coordination polymer (LCP) named UZAR-S12 was synthesized. Its structure and formula [Zn2(μ2-mMP)3(NO3)(DMF)(H2O)]n·nDMF (mMP, 6-(methylmercapto)purinate; DMF, N,N-dimethylformamide) were determined by single crystal X-ray diffraction. UZAR-S12 is characterized by two kinds of Zn centres. Zn1 is bonded to three mMP ligands at the basal plane, and one water molecule and one DMF molecule at the axial positions, giving rise to a slightly distorted trigonal bipyramidal geometry. Zn2 is coordinated to three mMP linkers and one NO3− resulting in a tetrahedral environment. The layers are arranged yielding a three-dimensional network with guest DMF trapped between them. Lattice and coordination DMF can be replaced reversibly by other solvents producing a contraction (methanol) or an expansion (N,N-diethylformamide) of the framework. A solvent exchange method was used from the vapour phase which preserved the sample crystallinity.

 Please wait while we load your content...
Please wait while we load your content...