Organic thin film transistors with novel photosensitive polyurethane as dielectric layer
Abstract
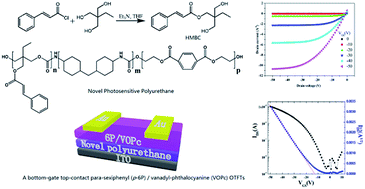
In this paper, we report the design, synthesis, and dielectric properties of a novel photosensitive polyurethane dielectric film used in organic thin-film transistors (OTFTs). The novel photosensitive polyurethane dielectric film is readily fabricated by spin coating followed by curing under UV light. The film exhibits excellent insulating properties, smooth surface, and excellent photosensitive properties. The bottom-gate top-contact para-sexiphenyl (p-6P)/vanadyl-phthalocyanine (VOPc) OTFTs with photosensitive polyurethane films as the dielectric layer exhibit excellent performance with a mobility of 0.13 cm2 V−1 s−1, on/off ratio of 104 and ultralow leakage.

 Please wait while we load your content...
Please wait while we load your content...