Assembly of TiO2/graphene with macroporous 3D network framework as an advanced anode material for Li-ion batteries
Fei Li,
Jianzhong Jiang,
Xinjing Wang,
Fan Liu,
Jinzuan Wang,
Yanwei Chen,
Sheng Han* and
Hualing Lin*
School of Chemical and Environmental Engineering, Shanghai Institute of Technology, Shanghai 201418, China. E-mail: hansheng654321@sina.com; Tel: +86 13524694909
First published on 17th December 2015
Abstract
Three-dimensional (3D) TiO2–graphene frameworks (TGFs) with macroporous architecture were fabricated through the in situ synthesis of TiO2 with the participation of graphene oxide followed by hydrothermal assembly. TGFs exhibited a 3D hierarchical porous architecture with mesopores (2.4 nm), macropores (10–30 μm) and a large specific surface area (196 m2 g−1), which not only provided contacts between the electrode material and the electrolyte but also increased the mass transport of Li-ions in the charge/discharge process. When it was used as a cathode material in Li-ion batteries, TGFs presented an excellent reversible specific capacity of 210 mA h g−1 at 100 mA g−1 and an outstanding reversible cycling stability (111 mA h g−1 after 500 cycles); even at the current density of 500 mA g−1, TGF also performed very well. This excellent electrochemical performance was attributed to the unique 3D hierarchical porous architecture and the synergistic effects of TiO2 and graphene in Li-ion storage and transport.
1. Introduction
Rechargeable Li-ion batteries (LIBs) have been investigated as the most promising power sources in portable electronic devices and electrical/hybrid vehicles.1,2 Devices with large reversible capacity, long cycle life, good rate performance, high safety and low-cost electrode materials are imperative to meet the increasing energy storage requirements.3–10 Titanium dioxide (TiO2) is one of the most extensively studied anode materials for its low volume change (less than 4%), prominent structural stability and environmental friendliness.11–13 In addition, the working voltage (1.7 V vs. Li/Li+) of TiO2 is much higher than that of the conventional carbon-based anode materials (below 0.2 V vs. Li/Li+).14,15 However, its poor ionic diffusivity and electronic conductivity (∼10–12 S cm−1) greatly limit its electrochemical properties as an electrode material.16,17 One of the feasible strategies is to construct nanostructured TiO2, such as nanowires, nanoparticles (NPs), nanotubes mesoporous nanospheres and nanorods.18–23 These nanostructured TiO2 materials can noticeably shorten the diffusion distances in the transport of both electrons and lithium-ions, remarkably increase the electrode/electrolyte contact region and effectively improve battery stability during the charge/discharge process. However, TiO2 nanocrystals may possibly shed from the electrode surface, cross the separator and cause internal short circuits.24 The other impactful strategy is to construct hybrid electrodes composed of TiO2 and conductive additives providing rapid electron channels.Graphene, with a two-dimensional (2D) single carbon atom layer, has attracted tremendous attentions because of its excellent electrical conductivity (∼15000 cm2 V−1 s−1), large specific surface area (∼2600 m2 g−1) and extraordinary mechanical properties.25,26 Furthermore, the flexible honeycomb structure provides conduction channels for electron transport and allows other nanoscale materials to fill the clearance space. Several TiO2/graphene nanocomposites (TGNs) that showed superior Li-storage performance have been reported.27,28 However, these TGNs reported were simply prepared by decorating TiO2 NPs or nanocrystals with specific morphologies onto the graphene or forming 2D TGN paper. Moreover, 3D graphene-based frameworks integrate individual 2D graphene sheets into continuous structures. Therefore, such 3D hybrids not only contain the ascendant properties of single component but also translate the intrinsic features into the macroscopic scale. In particular, the construction of 3D TiO2–graphene frameworks (TGFs) with macroporous architecture exhibit an interconnected graphene network to promote electrolyte infiltration, carriage of charge carriers and adjustment of electrode volume changes. Thus, TGF construction is a promising strategy to achieve highly reversible capacity and excellent rate performance for LIBs.29–31
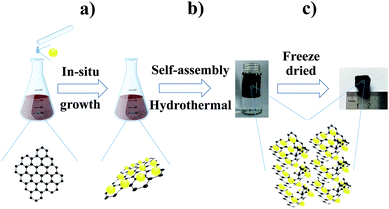
In this study, we prepared a novel self-assembly approach to construct 3D macroscopic TGFs as anode materials for LIBs. The 3D TGFs were synthesised by a two-step procedure, in which TiO2 was first decorated on graphene oxide (GO) sheets under mild conditions (80 °C) with stirring and then through hydrothermal treatment to establish 3D macroscopic frameworks. The obtained 3D TGFs showed high specific surface area, numerous macropores with diameters to be dozens of micrometres, and a large aspect ratio. In particular, this unique hybrid 3D architecture effectively accommodated the excessive volume change during the lithiation–delithiation process and provided numerous multidimensional channels for Li-ion diffusing from the electrolyte to the electrode. Serving as the anode materials for LIBs, TGFs not only exhibited an excellent reversible capacity of 210 mA h g−1 with up to 100 charge–discharge cycles at 100 mA g−1 but also maintained specific capacities of 82 mA h g−1 at a ultrahigh current density of 5000 mA g−1.
2. Experimental
2.1. TGF synthesis
GO was synthesised from natural graphite powder by using a modified Hummers method.32 Titanic sulphate (Ti(SO4)2·9H2O, 0.3 g) was first dissolved into 60 ml of dimethylformamide (DMF) suspension of GO (0.5 mg ml−1), and then 10 ml of deionised (DI) water was added, followed by ultrasonication for 30 min to form a homogeneous suspension. Subsequently, the mixture was kept at 80 °C with stirring for 12 h. The resulting suspension was then centrifuged and washed with distilled water thrice to remove the DMF. Afterward, the as-prepared GO nanosheets decorated with TiO2 NPs were dispersed into DI water (20 ml) in a cylindrical vial (30 ml), then they were transferred into an 80 ml Teflon-lined stainless steel autoclaves and hydrothermally treated at 180 °C for 12 h. Finally, the as-prepared sample was freeze-dried overnight and the TGF 3D monolithic architecture could be obtained. For comparison purposes, TGFs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and (2
2) and (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were fabricated by blending GO with different volumes of Ti(SO4)2·9H2O ranging from 0.15 to 0.45 g.
1) were fabricated by blending GO with different volumes of Ti(SO4)2·9H2O ranging from 0.15 to 0.45 g.
2.2. Material characterizations
The morphology and microstructures of the samples were characterised by field-emission scanning electron microscopy (FESEM) system (FEI, Sirion 200) and transmission electron microscopy (TEM, JEOL JEM-2010, 200 kV). Powder X-ray diffraction (XRD) pattern was collected by a Rigaku X-ray diffractometer with Cu-Kα irradiation (λ = 0.15406 Å). Raman spectroscopy was conducted on a SENTERRA instrument, with an 532 nm line Ar-ion laser (about 5 m W). Thermal gravimetric analysis (TGA) was monitored on a TA Q5000IR with a heating rate of 10 °C min−1 under flowing air. X-ray photoelectron spectroscopy was performed by using a PHI 5700 ESCA spectrometer with a monochromated Al Kα radiation source. The specific surface area was tested by the Brunauer–Emmett–Teller (BET) method at 77 K in N2 atmosphere with a Micrometrics SAP 2010 surface area apparatus.2.3. Electrochemical measurements
Electrochemical experiments were performed with standard 2016 coin-type cells. The as-synthesised TGFs were mixed with carbon black (Super-P) and poly-(vinyl difluoride) at a weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in N-methyl-2-pyrrolidone solvent (Aldrich, 99.5%) until they were mixed evenly enough to prepare the working electrodes. The resultant slurries were uniformly pasted onto Cu foils (99.6%) with an area of 1 cm2 and then dried in a vacuum oven at 60 °C for 12 h. The mass loading of the working electrode materials was about 0.8–1.2 mg cm−2. The electrolyte was 1 M LiPF6 in ethylene carbonate–dimethyl carbonate (1
1 in N-methyl-2-pyrrolidone solvent (Aldrich, 99.5%) until they were mixed evenly enough to prepare the working electrodes. The resultant slurries were uniformly pasted onto Cu foils (99.6%) with an area of 1 cm2 and then dried in a vacuum oven at 60 °C for 12 h. The mass loading of the working electrode materials was about 0.8–1.2 mg cm−2. The electrolyte was 1 M LiPF6 in ethylene carbonate–dimethyl carbonate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) obtained from Ube Industries, Ltd. Cells were assembled in an argon-filled glove box, where the concentration of water and oxygen was kept below 1 ppm. Galvanostatic charge–discharge cycling was performed using a battery cycler (LAND-CT2001A) with various rates from 100 mA g−1 to 5000 mA g−1 at a voltage range from 1.0 V to 3.0 V vs. Li/Li+. Electrochemical impedance spectroscopy measurements were conducted by applying a sine wave with a amplitude of 5.0 mV over the frequency range of 0.01 Hz to 100 kHz.
1 v/v) obtained from Ube Industries, Ltd. Cells were assembled in an argon-filled glove box, where the concentration of water and oxygen was kept below 1 ppm. Galvanostatic charge–discharge cycling was performed using a battery cycler (LAND-CT2001A) with various rates from 100 mA g−1 to 5000 mA g−1 at a voltage range from 1.0 V to 3.0 V vs. Li/Li+. Electrochemical impedance spectroscopy measurements were conducted by applying a sine wave with a amplitude of 5.0 mV over the frequency range of 0.01 Hz to 100 kHz.
3. Results and discussion
The fabrication procedure of TGF is schematically illustrated in Fig. 1. First, Ti4+ cations from Ti(SO4)2·9H2O can favourably intermix with oxygen-containing groups on GO sheets through electrostatic interactions. Second, when the temperature reached 80 °C, the Ti4+ started to form Ti(OH)4 through stepwise hydrolysis deposited on the surface of the GO sheets. Finally, as the basic building blocks of the macroporous hybrid, 2D GO sheets with a uniform decoration of Ti(OH)4 self-assembled into 3D monolithic networks through hydrothermal treatment. During the last procedure, Ti(OH)4 was transformed into TiO2, and the GO simultaneously transformed into reduced graphene oxide (RGO) then the TGF was obtained. Specifically, TGFs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), TGFs (1
2), TGFs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and TGFs (2
1), and TGFs (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were fabricated by blenging GO with different volumes of Ti(SO4)2·9H2O insuring the weight ratios of GO
1) were fabricated by blenging GO with different volumes of Ti(SO4)2·9H2O insuring the weight ratios of GO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti to be 1
Ti to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 respectively, in the first step of the synthesis.
1 respectively, in the first step of the synthesis.
The morphologies and microstructure of the as-prepared TGF were first investigated using FESEM and TEM (Fig. 2). The FESEM images of a cross-section of TGF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) exhibited a highly interconnected 3D TGFs, which contained uniform macropores with diameters ranging from 10 μm to 30 μm (Fig. 2a). The high-resolution FESEM images of TGF apparently exposed that TiO2 NPs were consistently loaded on both sides of the graphene sheets without free particles or unloaded graphene sheets (Fig. 2b). Elemental mapping images of TGF confirmed the presence of Ti and O components in TGF (Fig. 2c), which further revealed that TiO2 homogeneously adhered to the surface of graphene sheets. Moreover, the high crystallinity of TiO2 shown in the TEM images demonstrated that TiO2 NPs with diameters of 5–7 nm were deposited on the graphene sheet surface (Fig. 2d). As shown in Fig. 2e, the d-spacing of the lattice fringes was about 0.34 nm, which could be identified by the interlayer spacing of the (101) plane in anatase TiO2 crystal lattice in accordance with the results of the XRD measurements.33
1) exhibited a highly interconnected 3D TGFs, which contained uniform macropores with diameters ranging from 10 μm to 30 μm (Fig. 2a). The high-resolution FESEM images of TGF apparently exposed that TiO2 NPs were consistently loaded on both sides of the graphene sheets without free particles or unloaded graphene sheets (Fig. 2b). Elemental mapping images of TGF confirmed the presence of Ti and O components in TGF (Fig. 2c), which further revealed that TiO2 homogeneously adhered to the surface of graphene sheets. Moreover, the high crystallinity of TiO2 shown in the TEM images demonstrated that TiO2 NPs with diameters of 5–7 nm were deposited on the graphene sheet surface (Fig. 2d). As shown in Fig. 2e, the d-spacing of the lattice fringes was about 0.34 nm, which could be identified by the interlayer spacing of the (101) plane in anatase TiO2 crystal lattice in accordance with the results of the XRD measurements.33
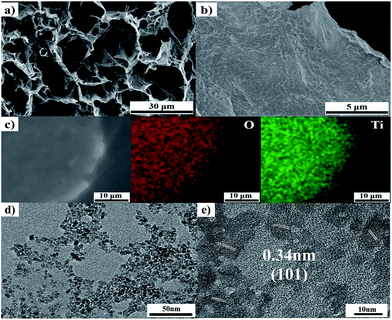 | ||
Fig. 2 (a) and (b) FESEM images of TGF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); (c) element-mapping image of TGF; and (d) and (e) TEM images of TGF. 1); (c) element-mapping image of TGF; and (d) and (e) TEM images of TGF. | ||
Powder XRD experiment was conducted to gain the internal structure of the TGF, RGO and GO (Fig. 3a). The obvious peaks at about 2θ = 25.1°, 37.5°, 47.8°, 53.9°, 62.3°, 69.1° and 74.8° could be attributed to the (101), (004), (200), (105), (213), (116) and (215), respectively, which were well-indexed to the anatase TiO2 (JCPDS card 75-1537), demonstrating that GO application didn't influence the crystal fabrication of TiO2. At 26.6°, no apparent diffraction peak was observed, suggesting that no further agglomeration of few-layered RGO sheets hindered by TiO2 NPs and that the graphene sheets were effectively separated and highly dispersed into the TiO2 matrix.34
The Raman spectra of TGF, TiO2 and GO were shown in Fig. 3b. The peaks at 153, 202, 394, 505 and 631 cm−1 were attributed to the Raman modes of Eg (1), Eg (1), B1g (1), A1g and Eg (3), respectively, showing increased broadening and systematic frequency shifts in comparison to bulk anatase TiO2. Such Raman signal broadening and frequency shifting trends on the dimension was less than the typical 20 nm, supported by the TEM image.35,36 The G band (about 1595 cm−1) identified with the in-plane vibration of sp2-hybridised carbon and the D band (about 1345 cm−1) originating from chaotic carbon owing to RGO.37 After hydrothermal treatment, the D/G intensity ratio of the TGF composite (ID/IG = 1.02) was larger than that of the GO (ID/IG = 0.95), which indicated a decrease in the average size of the sp2 domains and an increase in the disorder degree of the graphene.38
TGA (in air from 25–800 °C with a heating rate of 20 °C min−1) was performed to determine the chemical composition of TGFs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), (1
2), (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and (2
1) and (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Fig. 3c). As shown in the TGA results, the TGFs lost weight in three steps; first, between 25 and 100 °C; then, between 100 and 400 °C; and finally, between 400 and 550 °C. The weight loss (<5%) appeared below 150 °C was attributed to the evaporation of adsorbed water molecules. The major weight loss from 100 °C to 550 °C was owing to the graphene combustion. In particular, the TGF curve showed a significant weight loss at approximately 400 °C and a constant weight at above 500 °C, indicating that TiO2 could improve the thermal stability of the graphene by conglutinating on its surface. Based on the calculations, the TiO2 contents in TGFs (1
1) (Fig. 3c). As shown in the TGA results, the TGFs lost weight in three steps; first, between 25 and 100 °C; then, between 100 and 400 °C; and finally, between 400 and 550 °C. The weight loss (<5%) appeared below 150 °C was attributed to the evaporation of adsorbed water molecules. The major weight loss from 100 °C to 550 °C was owing to the graphene combustion. In particular, the TGF curve showed a significant weight loss at approximately 400 °C and a constant weight at above 500 °C, indicating that TiO2 could improve the thermal stability of the graphene by conglutinating on its surface. Based on the calculations, the TiO2 contents in TGFs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), (1
2), (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and (2
1) and (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were approximately 61.9%, 72.2% and 76.2%, respectively.
1) were approximately 61.9%, 72.2% and 76.2%, respectively.
The porous features of TGF were further investigated using the N2 adsorption–desorption analysis (Fig. 3d). The BET surface area of TGF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), (1
2), (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and (2
1) and (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) nanocomposites were calculated to be as high as 220, 196 and 168 m2 g−1, respectively. The specific surface area of the TGF nanocomposites was mainly due to the contribution of graphene, which highlighted that the building up of 3D frameworks was an effective way to achieve a high specific surface area for hybrid materials. Furthermore, the adsorption–desorption isotherm of all the three samples exhibited the typical-type IV nitrogen adsorption branch with an H2 hysteresis loop, suggesting the existence of mesoporous structures in the hybrid.39 The total pore volume was 0.1288, 0.1174 and 0.1082 cm3 g−1 with an average pore diameter to be 2.34, 2.40 and 2.57 nm, respectively, based on the Barrett– Joyner–Halenda calculations. Such porous structure could not only provide extreme contacts between the electrode material and the electrolyte but also enhance the mass transport of Li-ions in charge/discharge process, which was crucial to the rate capability of LIBs.
1) nanocomposites were calculated to be as high as 220, 196 and 168 m2 g−1, respectively. The specific surface area of the TGF nanocomposites was mainly due to the contribution of graphene, which highlighted that the building up of 3D frameworks was an effective way to achieve a high specific surface area for hybrid materials. Furthermore, the adsorption–desorption isotherm of all the three samples exhibited the typical-type IV nitrogen adsorption branch with an H2 hysteresis loop, suggesting the existence of mesoporous structures in the hybrid.39 The total pore volume was 0.1288, 0.1174 and 0.1082 cm3 g−1 with an average pore diameter to be 2.34, 2.40 and 2.57 nm, respectively, based on the Barrett– Joyner–Halenda calculations. Such porous structure could not only provide extreme contacts between the electrode material and the electrolyte but also enhance the mass transport of Li-ions in charge/discharge process, which was crucial to the rate capability of LIBs.
X-ray photoelectron spectroscopy (XPS) was employed to gain the insight chemical bonding environment of Ti, O, and C atoms within TGF (take the TGF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for example). The peaks of Ti, O, and C elements peaks could be observed in the full spectra of TGF (Fig. 4a). The binding energy (BE) peaks of Ti 2p1/2 and Ti 2p3/2 for TGF was positioned at 459.1 and 464.8 eV (Fig. 4c) with a peak separation of 5.7 eV. These peak locations and separation were in excellent agreement with the reported values for fully oxidized anatase TiO2 single crystals.40,41 Additionally, the components peaks of TGF at 530.3 eV (O–Ti), 531.7 eV (C
1) for example). The peaks of Ti, O, and C elements peaks could be observed in the full spectra of TGF (Fig. 4a). The binding energy (BE) peaks of Ti 2p1/2 and Ti 2p3/2 for TGF was positioned at 459.1 and 464.8 eV (Fig. 4c) with a peak separation of 5.7 eV. These peak locations and separation were in excellent agreement with the reported values for fully oxidized anatase TiO2 single crystals.40,41 Additionally, the components peaks of TGF at 530.3 eV (O–Ti), 531.7 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and 532.8 eV (C–O) could be divided from the O 1s spectra for the compositions (Fig. 4d).42 The C 1s XPS spectrum of GO (Fig. 4b) could be deconvoluted into three peaks (centered at 284.8 eV, 286.9 eV and 288.6 eV), which were ascribed to sp2 bonded carbon (C–C), epoxy/hydroxyls (C–O), and carboxyl (O–C
O) and 532.8 eV (C–O) could be divided from the O 1s spectra for the compositions (Fig. 4d).42 The C 1s XPS spectrum of GO (Fig. 4b) could be deconvoluted into three peaks (centered at 284.8 eV, 286.9 eV and 288.6 eV), which were ascribed to sp2 bonded carbon (C–C), epoxy/hydroxyls (C–O), and carboxyl (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), respectively, indicating the high percentage of oxygen containing functional groups.43,44 In comparison, in the C 1s XPS spectrum of the TGF (Fig. 4b), the peaks for C–C, C–O and O–C
O), respectively, indicating the high percentage of oxygen containing functional groups.43,44 In comparison, in the C 1s XPS spectrum of the TGF (Fig. 4b), the peaks for C–C, C–O and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O were much lower in intensity than those in GO, indicating the efficient deoxygenation and reduction during the hydrothermal process.45
O were much lower in intensity than those in GO, indicating the efficient deoxygenation and reduction during the hydrothermal process.45
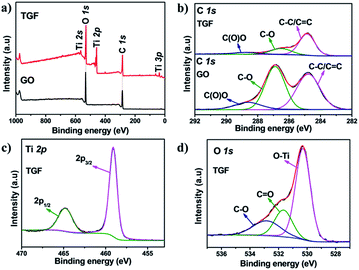 | ||
| Fig. 4 (a) XPS spectrum of TGF and GO; (b) XPS C 1s spectrum of the TGF and GO; (c) Ti 2p and (d) O 1s XPS spectra of TGF. | ||
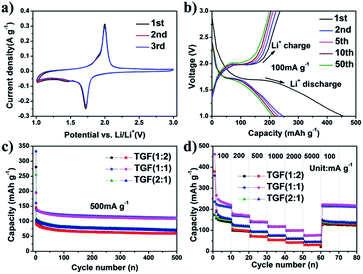
Cyclic voltammetry (CV) was performed at a scan rate of 0.1 mV s−1 in the voltage range of 1.0–3.0 V to investigate the electrochemical reactivity of the TGF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) hybrids, as shown in Fig. 5a. The CV curves clearly observed from the TGF (1
1) hybrids, as shown in Fig. 5a. The CV curves clearly observed from the TGF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) showed a pair of redox peaks, including a reduction peak at 1.72 V and an oxidation peak at 1.99 V, which were corresponding to the Li insertion/extraction in anatase TiO2 lattice.46,47 As it was showed in Fig. 5a, the cathodic and corresponding anodic peaks only displayed a small change during the three initial CVs, suggesting little capacity loss. This result indicated that the TGF (1
1) showed a pair of redox peaks, including a reduction peak at 1.72 V and an oxidation peak at 1.99 V, which were corresponding to the Li insertion/extraction in anatase TiO2 lattice.46,47 As it was showed in Fig. 5a, the cathodic and corresponding anodic peaks only displayed a small change during the three initial CVs, suggesting little capacity loss. This result indicated that the TGF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) nanocomposite showed a higher reversibility in electrochemical reactions.
1) nanocomposite showed a higher reversibility in electrochemical reactions.
The electrochemical performances of TGF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were evaluated by galvanostatic charge–discharge cycling in a cell using Li metal as the counter electrode, in the voltage range of 1.0–3.0 V. Fig. 5b exhibited the charge–discharge curves of TGF (1
1) were evaluated by galvanostatic charge–discharge cycling in a cell using Li metal as the counter electrode, in the voltage range of 1.0–3.0 V. Fig. 5b exhibited the charge–discharge curves of TGF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at a current density of 100 mA g−1, in which discharge and charge potentials stabilised at ∼1.73 and ∼1.98 V appeared, and these curves were consistent with the CV results. The first discharge and charge cycle capacities were 457 and 236 mA h g−1, respectively, which corresponded to an irreversible capacity loss of 48%. This strong reduction capacity was mainly caused by the electrolyte decomposition on the new surface of the carbon material and the formation of a solid electrolyte interphase (SEI) layer on the electrode surface during the first discharge,24 which could also be confirmed by electrochemical impedance spectroscopy (EIS) (see details below). For the second cycle, the charge and discharge capacities were 247 and 222 mA h g−1, indicating a reduced irreversible capacity loss of only about 10%. Moreover, the whole cycle charge curve displayed a similar tendency, suggesting that the charge/discharge process was highly reversible.
1) at a current density of 100 mA g−1, in which discharge and charge potentials stabilised at ∼1.73 and ∼1.98 V appeared, and these curves were consistent with the CV results. The first discharge and charge cycle capacities were 457 and 236 mA h g−1, respectively, which corresponded to an irreversible capacity loss of 48%. This strong reduction capacity was mainly caused by the electrolyte decomposition on the new surface of the carbon material and the formation of a solid electrolyte interphase (SEI) layer on the electrode surface during the first discharge,24 which could also be confirmed by electrochemical impedance spectroscopy (EIS) (see details below). For the second cycle, the charge and discharge capacities were 247 and 222 mA h g−1, indicating a reduced irreversible capacity loss of only about 10%. Moreover, the whole cycle charge curve displayed a similar tendency, suggesting that the charge/discharge process was highly reversible.
The long cycling performance of TGF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), (1
2), (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and (2
1) and (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) electrodes was compared at a constant current density of 500 mA g−1 between 1.0 and 3.0 V (Fig. 5c). All three samples exhibited excellent stability during the cycle processes, and TGF (1
1) electrodes was compared at a constant current density of 500 mA g−1 between 1.0 and 3.0 V (Fig. 5c). All three samples exhibited excellent stability during the cycle processes, and TGF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) displayed the highest capacity. The first charge and discharge capacities of the TGF (1
1) displayed the highest capacity. The first charge and discharge capacities of the TGF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) electrode were 332 and 195 mA h g−1, respectively, followed by a slight decrease in the capacity during the initial 50 cycles. More importantly, a high capacity of 111 mA h g−1 was maintained until 500 cycles, implying that the long-term charge/discharge cycles did not severely spoil the structure of the TGFs and the high discharge capacity could be well preserved. Comparatively, the reversible capacities of TGF (1
1) electrode were 332 and 195 mA h g−1, respectively, followed by a slight decrease in the capacity during the initial 50 cycles. More importantly, a high capacity of 111 mA h g−1 was maintained until 500 cycles, implying that the long-term charge/discharge cycles did not severely spoil the structure of the TGFs and the high discharge capacity could be well preserved. Comparatively, the reversible capacities of TGF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and (2
2) and (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) after 500 cycles were 60 and 70 mA h g−1, which were lower than that of TGF (1
1) after 500 cycles were 60 and 70 mA h g−1, which were lower than that of TGF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
The high-rate performance of the TGF electrode was also investigated at various current densities between 1.0 and 3.0 V to inspect the possibility for battery applications. As indicated in Fig. 5d, the first discharge–charge step delivered a specific discharge capacity of 462 mA h g−1. When cycled at 100 mA g−1, the TGF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) delivered a second discharge capacity of 270 mA h g−1 and dropped to near 222 mA h g−1 after 10 cycles. During the subsequent cycles, the reversible capacities of the TGF (1
1) delivered a second discharge capacity of 270 mA h g−1 and dropped to near 222 mA h g−1 after 10 cycles. During the subsequent cycles, the reversible capacities of the TGF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) electrode were 185, 150, 125 and 105 mA h g−1 at current rates of 200, 500, 1000 and 2000 mA g−1, respectively. Even at super high current densities of 5000 mA g−1, the homologous recharge capacities of the TGF (1
1) electrode were 185, 150, 125 and 105 mA h g−1 at current rates of 200, 500, 1000 and 2000 mA g−1, respectively. Even at super high current densities of 5000 mA g−1, the homologous recharge capacities of the TGF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) electrode retained 82 mA h g−1 with nearly 100% coulombic efficiency, which clearly demonstrated that the TGF (1
1) electrode retained 82 mA h g−1 with nearly 100% coulombic efficiency, which clearly demonstrated that the TGF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) nanocomposite could tolerate varied discharge current densities. Such remarkable rate capability and high capacity of the TGF nanocomposite may be attributed to the high electronic conductivity of graphene and the mesoporous structures of the TGF nanocomposite.43 Moreover, after the high-current density measurements, the capacity of the TGF (1
1) nanocomposite could tolerate varied discharge current densities. Such remarkable rate capability and high capacity of the TGF nanocomposite may be attributed to the high electronic conductivity of graphene and the mesoporous structures of the TGF nanocomposite.43 Moreover, after the high-current density measurements, the capacity of the TGF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) nanocomposite at 100 mA g−1 could recover to the initial value (221 mA h g−1), indicating an excellent cycle performance. For comparison, the performance of TGF (1
1) nanocomposite at 100 mA g−1 could recover to the initial value (221 mA h g−1), indicating an excellent cycle performance. For comparison, the performance of TGF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and (2
2) and (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) electrodes were only 132 and 150 mA h g−1, respectively, at a current density of 100 mA g−1 after 80 cycles. The capacity of TGF (1
1) electrodes were only 132 and 150 mA h g−1, respectively, at a current density of 100 mA g−1 after 80 cycles. The capacity of TGF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) achieved in this study was superior to that of the previous reports, in which the TiO2/graphene and other TiO2/carbon composites owned a similar TiO2 weight content.
1) achieved in this study was superior to that of the previous reports, in which the TiO2/graphene and other TiO2/carbon composites owned a similar TiO2 weight content.
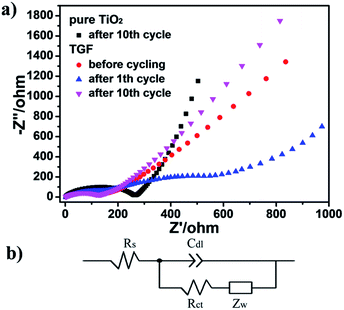
To gain an insight vision of the prominent electrochemical behavior of the TGF, electrochemical impedance spectroscopy (EIS) measurements of pure TiO2 and TGF electrode were performed (Fig. 6a). Nyquist plots showed only one semicircle in the high-frequency range and an inclined line in the low-frequency range for TGF before cycling and after the 10th cycle, which were assigned to the charge-transfer impedance in the electrode/electrolyte interface and the Li+ ion diffusion process, respectively. However, there were two semicircles and a line which could be found in the EIS profile indicating the formation of solid–electrolyte-interphase (SEI) films after the 1st cycle.48,49 After the 10th cycle, the diameter of the semicircle for TGF was much smaller than that for pure TiO2, which suggested that TGF possessed lower contact and charge-transfer resistances. This result could be further supported by simulating their kinetic parameters through the typical Randles equivalent circuit (Fig. 6b). In contrast, Rct value of the TGF electrode was 62.29 Ω which was significantly lower than those of pure TiO2 electrode (137.01 Ω). Furthermore, the linear Warburg regions at low frequency in Nyquist plots could be used to represent the Li-ion diffusion behavior and the diffusion coefficient of Li+ also calculated by the following formula (eqn (1)):48
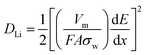 | (1) |
4. Conclusions
A self-assembly approach was developed to construct 3D macroscopic TGF nanostructures, which could be suitably used as high-performance anode materials for LIBs. The integration of graphene and TiO2 in the TGF architecture facilitated homogeneous dispersion, high surface area and porous structures. In particular, the interconnected macropores could efficiently form the contact with electrolyte and supply open channels for electron transport, leading to the increased rate capability and prominent cycle stability even at a high current density. Moreover, this synthetic strategy provided an extendible way to fabricate 3D metal (oxide)–graphene nanocomposites for various applications, such as Li-ion batteries, catalysts and sensors.Acknowledgements
This project was supported by the Innovation Program of Shanghai Municipal Education Commission (Project Number 09YZ387), Innovation Program of Shanghai Municipal Education Commission (Project Number 11ZZ179), Science and Technology Commission of Shanghai Municipality (Project Number 09QT1400600), ShuGuang Project (Project Number 11SG54), National Natural Science Foundation of China (Project Number 20976105) and Shanghai Leading Academic Discipline Project (Project Number J51503).Notes and references
- K. Amine, I. Belharouak, Z. Chen, T. Tran, H. Yumoto, N. Ota, S. T. Myung and Y. K. Sun, Adv. Mater., 2010, 22, 3052–3057 CrossRef CAS PubMed.
- J. Hassoun, K.-S. Lee, Y.-K. Sun and B. Scrosati, J. Am. Chem. Soc., 2011, 133, 3139–3143 CrossRef CAS PubMed.
- Y. Idota, T. Kubota, A. Matsufuji, Y. Maekawa and T. Miyasaka, Science, 1997, 276, 1395–1397 CrossRef CAS.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. Tarascon, Nature, 2000, 407, 496–499 CrossRef CAS PubMed.
- N. Recham, J.-N. Chotard, L. Dupont, C. Delacourt, W. Walker, M. Armand and J.-M. Tarascon, Nat. Mater., 2010, 9, 68–74 CrossRef CAS PubMed.
- R. Bhattacharyya, B. Key, H. Chen, A. S. Best, A. F. Hollenkamp and C. P. Grey, Nat. Mater., 2010, 9, 504–510 CrossRef CAS PubMed.
- D. Wu, F. Zhang, H. Liang and X. Feng, Chem. Soc. Rev., 2012, 41, 6160–6177 RSC.
- S. Yang, X. Feng, S. Ivanovici and K. Müllen, Angew. Chem., Int. Ed., 2010, 49, 8408–8411 CrossRef CAS PubMed.
- B. Luo, B. Wang, M. Liang, J. Ning, X. Li and L. Zhi, Adv. Mater., 2012, 24, 1405–1409 CrossRef CAS PubMed.
- B. Luo, Y. Fang, B. Wang, J. Zhou, H. Song and L. Zhi, Energy Environ. Sci., 2012, 5, 5226–5230 CAS.
- C. Liu, F. Li, L. P. Ma and H. M. Cheng, Adv. Mater., 2010, 22, E28–E62 CrossRef CAS PubMed.
- J. B. Goodenough and Y. Kim, Chem. Mater., 2009, 22, 587–603 CrossRef.
- E. Yoo, J. Kim, E. Hosono, H.-s. Zhou, T. Kudo and I. Honma, Nano Lett., 2008, 8, 2277–2282 CrossRef CAS PubMed.
- S. Yang, X. Feng and K. Müllen, Adv. Mater., 2011, 23, 3575–3579 CrossRef CAS PubMed.
- J. S. Chen, Y. L. Tan, C. M. Li, Y. L. Cheah, D. Luan, S. Madhavi, F. Y. C. Boey, L. A. Archer and X. W. Lou, J. Am. Chem. Soc., 2010, 132, 6124–6130 CrossRef CAS PubMed.
- T. Fröschl, U. Hörmann, P. Kubiak, G. Kučerová, M. Pfanzelt, C. K. Weiss, R. Behm, N. Hüsing, U. Kaiser and K. Landfester, Chem. Soc. Rev., 2012, 41, 5313–5360 RSC.
- H. B. Wu, J. S. Chen, H. H. Hng and X. W. D. Lou, Nanoscale, 2012, 4, 2526–2542 RSC.
- J. Ye, W. Liu, J. Cai, S. Chen, X. Zhao, H. Zhou and L. Qi, J. Am. Chem. Soc., 2010, 133, 933–940 CrossRef PubMed.
- F. Wu, Z. Wang, X. Li and H. Guo, J. Mater. Chem., 2011, 21, 12675–12681 RSC.
- M.-Z. Ge, S.-H. Li, J.-Y. Huang, K.-Q. Zhang, S. S. Al-Deyab and Y.-K. Lai, J. Mater. Chem. A, 2015, 3, 3491–3499 CAS.
- L. He, R. Ma, N. Du, J. Ren, T. Wong, Y. Li and S. T. Lee, J. Mater. Chem., 2012, 22, 19061–19066 RSC.
- G. Zhang, H. B. Wu, T. Song, U. Paik and X. W. D. Lou, Angew. Chem., Int. Ed., 2014, 53, 12590–12593 CAS.
- R. Gao, B. Cao, Y. Hu, Z. Feng, D. Wang, W. Hu, J. Chen, Z. Jie, H. Qiu and K. Xu, N. Engl. J. Med., 2013, 368, 1888–1897 CrossRef CAS PubMed.
- L. Song, L. Li, X. Gao, J. Zhao, T. Lu and Z. Liu, J. Mater. Chem. A, 2015, 3, 6862–6872 CAS.
- L. Chen, Y. Zhou, W. Tu, Z. Li, C. Bao, H. Dai, T. Yu, J. Liu and Z. Zou, Nanoscale, 2013, 5, 3481–3485 RSC.
- X. Fan, W. Peng, Y. Li, X. Li, S. Wang, G. Zhang and F. Zhang, Adv. Mater., 2008, 20, 4490–4493 CrossRef CAS.
- M. Zhen, L. Su, Z. Yuan, L. Liu and Z. Zhou, RSC Adv., 2013, 3, 13696–13701 RSC.
- Y. Gan, L. Zhu, H. Qin, Y. Xia, H. Xiao, L. Xu, L. Ruan, C. Liang, X. Tao and H. Huang, Solid State Ionics, 2015, 269, 44–50 CrossRef CAS.
- Z. Chen, W. Ren, L. Gao, B. Liu, S. Pei and H.-M. Cheng, Nat. Mater., 2011, 10, 424–428 CrossRef CAS PubMed.
- S. Ding, J. S. Chen, D. Luan, F. Y. C. Boey, S. Madhavi and X. W. D. Lou, Chem. Commun., 2011, 47, 5780–5782 RSC.
- W. Chen, S. Li, C. Chen and L. Yan, Adv. Mater., 2011, 23, 5679–5683 CrossRef CAS PubMed.
- W. S. Hummers Jr and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef.
- Z. Ma, Y. Fan, G. Shao, G. Wang, J. Song and T. Liu, ACS Appl. Mater. Interfaces, 2015, 7, 2937–2943 CAS.
- B. Li, H. Cao, J. Shao and M. Qu, Chem. Commun., 2011, 47, 10374–10376 RSC.
- C. Liu, H. Sun and S. Yang, Chem.–Eur. J., 2010, 16, 4381–4393 CrossRef CAS PubMed.
- U. Woggon, Optical properties of semiconductor quantum dots, Springer, 1997 Search PubMed.
- Z. H. Ni, T. Yu, Y. H. Lu, Y. Y. Wang, Y. P. Feng and Z. X. Shen, ACS Nano, 2008, 2, 2301–2305 CrossRef CAS PubMed.
- H. Xiang, Z. Li, K. Xie, J. Jiang, J. Chen, P. Lian, J. Wu, Y. Yu and H. Wang, RSC Adv., 2012, 2, 6792–6799 RSC.
- H. Liu, Z. Bi, X. G. Sun, R. R. Unocic, M. P. Paranthaman, S. Dai and G. M. Brown, Adv. Mater., 2011, 23, 3450–3454 CrossRef CAS PubMed.
- J. Yu, B. Yang and B. Cheng, Nanoscale, 2012, 4, 2670–2677 RSC.
- Z. Liu and B. Han, Adv. Mater., 2009, 21, 825–829 CrossRef CAS.
- J. Qiu, C. Lai, Y. Wang, S. Li and S. Zhang, Chem. Eng. J., 2014, 256, 247–254 CrossRef CAS.
- W. Li, F. Wang, Y. Liu, J. Wang, J. Yang, L. Zhang, A. A. Elzatahry, D. Al-Dahyan, Y. Xia and D. Zhao, Nano Lett., 2015, 15, 2186–2193 CrossRef CAS PubMed.
- C. Gong, Y. Zhang, M. Yao, Y. Wei, Q. Li, B. Liu, R. Liu, Z. Yao, T. Cui and B. Zou, RSC Adv., 2015, 5, 39746–39751 RSC.
- S. Li, M. Ling, J. Qiu, J. Han and S. Zhang, J. Mater. Chem. A, 2015, 3, 9700–9706 CAS.
- T. Hu, X. Sun, H. Sun, M. Yu, F. Lu, C. Liu and J. Lian, Carbon, 2013, 51, 322–326 CrossRef CAS.
- S. Liu, H. Jia, L. Han, J. Wang, P. Gao, D. Xu, J. Yang and S. Che, Adv. Mater., 2012, 24, 3201–3204 CrossRef CAS PubMed.
- M. Zhen, X. Guo, G. Gao, Z. Zhou and L. Liu, Chem. Commun., 2014, 50, 11915–11918 RSC.
- L. Su, Z. Zhou and M. Ren, Chem. Commun., 2010, 46, 2590–2592 RSC.
- Y. Wang, H. Liu, K. Wang, H. Eiji, Y. Wang and H. Zhou, J. Mater. Chem., 2009, 19, 6789–6795 RSC.
| This journal is © The Royal Society of Chemistry 2016 |