Tunable photocatalytic and photoelectric properties of I−-doped BiOBr photocatalyst: dramatic pH effect†
Changjiang Bia,
Jing Cao*ab,
Haili Lina,
Yunjian Wang*a and
Shifu Chenac
aCollege of Chemistry and Materials Science, Huaibei Normal University, Huaibei, 235000, Anhui, PR China. E-mail: caojing@mail.ipc.ac.cn; yjwang2013@gmail.com; Fax: +86 561 3806281; Tel: +86 561 3806281
bAnhui Collaborative Innovation Center of Advanced Functional Composite, Huaibei, 235000, Anhui, PR China
cCollege of Chemistry and Materials Engineering, Anhui Science and Technology University, Fengyang, 233100, Anhui, P R China
First published on 15th January 2016
Abstract
A series of I−-doped BiOBr (I-BiOBr) photocatalysts were prepared through adjusting the synthesis pH values of the reaction solution. The crystalline structure, light absorption, morphology and element component were measured by XRD, DRS, SEM, TEM, EDS, ICP-OES and XPS technologies. The doping of I− ions strongly broadened the visible light absorption range and improved the activity of I-BiOBr. More importantly, the photocatalytic activity of I-BiOBr dramatically depended on the synthesis pH values, in which I-BiOBr (0.5) displayed the best performance for removing methyl orange and phenol under visible light. The photoelectric property measurement revealed that I-BiOBr had stronger electron–hole separation efficiency than the corresponding BiOBr reference samples. With increase in the pH values, the I− ion doping contents varied a little but the surface charge state changed regularly. The decrease in the surface adsorbed negative Br− ions reduced the formation efficiency of active Br0 and resulted in low activity of I-BiOBr. This study provides a facile and efficient way to control the photocatalytic and photoelectric behaviors of novel I−-doped BiOX photocatalysts via changing the pH values of the reaction solution during synthesis.
1. Introduction
Semiconductor photocatalysis has been considered as the most potential strategy to solve the problems of energy crisis and environmental pollution.1–3 To exhibit the optimal ability of photocatalysis, constructing highly efficient and broadband spectrum semiconductor photocatalysts is gradually becoming the key procedure.4,5Except for the traditional metallic oxides6,7 and metallic sulfides,8,9 as a new type of layered ternary oxide semiconductor, bismuth oxyhalide (BiOX, X = Cl, Br, and I) has attracted extensive attention for its good photocatalytic performance and high stability.10 Among BiOCl, BiOBr and BiOI, BiOCl exhibits no visible light activity owing to its wide band gap energy (Eg = 3.5 eV)11 and BiOI exhibits a fast recombination rate of photocarriers.12 Comparatively, BiOBr possesses values between BiOCl and BiOI; however, the weaker visible light absorption ability (Eg = 2.7 eV) still hinders enhancement in its activity.13 To improve its visible light absorption, many effective ways have been exploited such as follows: (1) importing a narrow-band-gap semiconductor into BiOBr for generating a heterojunction-type system, such as BiOBr/BiOI,14 AgBr/BiOBr,15 Ag2CO3/BiOBr16 and g-C3N4/BiOBr;17,18 (2) depositing metal nanoparticles, such as Ag,19,20 Rh, Pd, and Pt;21 (3) in addition, as a direct useful way, ion doping has been widely used through generating a level of impurity between the valence band (VB) and the conduction band (CB) in the semiconductor crystal.22 As for BiOBr, the metallic ions Al, Fe, Ti, and Eu and the nonmetallic ions S, N, and I were successfully embedded in the interlayer between Br− and [Bi2O2]2+.23–29 As a result, ion doping not only strengthened the visible light utilization but also enhanced the photocatalytic activity of BiOBr.30 However, to date, few results have been reported concerning the ion doping efficiency and related mechanism for an ion doped BiOBr system. As is well known, the I− ion has a larger ion radius than the Br− ion, suggesting that I− has great potential as a dopant for BiOBr.31,32 Further research ensured that I− ions significantly enhanced the activity of BiOBr owing to the elevated VB potential.33
Generally, the ion doping process is facile but is easily influenced by various synthesis factors, which finally affect the apparent photocatalytic activity of the product. For example, the pH values of the reaction solutions greatly impact the crystalline phase, morphology, crystal size and surface area of photocatalysts.34–36 Therefore, pH modulation has been regarded as an important factor for regulating the activity of photocatalysts.
It is well known that the preparation of BiOX must be carried out in a suitably acidic solution. The reaction process can be summarized as proposed in eqn (1) and (2):
| Bi(NO3)3 + H2O → BiONO3 + 2HNO3 | (1) |
| BiONO3 + NaX → BiOX + NaNO3 | (2) |
Undoubtedly, the pH value during the synthesis controls the hydrolysis rate of Bi(NO3)3 to produce the BiONO3 precipitate, which then affects the final generation of BiOX. Thus, it is predictable that a significant pH effect is certainly present for ion doped BiOX. Considering the crucial role of pH value, it is necessary to investigate the relationship between the photocatalytic activity and the pH value of the reaction solution. However, to the best of our knowledge, the effects of the pH value on the photocatalytic activity of ion doped BiOX has not been reported to date.
In this study, for the first time, we prepared a series of novel I−-doped BiOBr (I-BiOBr) photocatalysts with different acidic solutions. The effects of pH value have been demonstrated by the photocatalytic activity and photoelectric property of the as-prepared I-BiOBr. On the basis of the abovementioned analysis, the activity variation mechanism has been discussed in detail with increase in the pH values. This is a facile strategy for efficiently regulating the photocatalytic activity of ion doped BiOX via changing the pH values of the reaction solution during its synthesis.
2. Experimental
2.1. Preparation of I-BiOBr samples
All the reagents were of analytical grade and obtained from Sinopharm Chemical Reagent Co., Ltd. (China), and used without further purification. Deionized water was used throughout this study. I-BiOBr was prepared through a facile chemical precipitation method at room temperature. First, 3.182 g Bi(NO3)3·5H2O was dissolved in 40 mL glacial acetic acid solution (pH = 1.96). Then, 5.0 mL mixed solution of 1.181 mol L−1 NaBr and 0.131 mol L−1 KI (the theoretical doping amounts of I− ions was 10%) was slowly added to the abovementioned solution. After the pH values of the reaction solution were adjusted to 0.5, 1.0, 2.0, 3.0, 4.0, 5.0 and 6.0 using ammonia water and persistently stirred for 3 h, the yellow precipitate obtained was filtered, washed and dried at 60 °C for 12 h. BiOBr precipitates used as the reference samples were also synthesized similarly to the abovementioned I-BiOBr samples, without adding KI solution.2.2. Characterization of catalyst
X-ray powder diffraction (XRD) analysis was carried out on a BRUKER D8 ADVANCE X-ray powder diffractometer with Cu Kα radiation (λ = 1.5406 Å) at 40 kV and 40 mA. UV-vis diffuse reflectance spectra (DRS) were collected using a TU-1901 UV-vis spectrophotometer (Beijing Purkinje General Instrument Co., Ltd.) equipped with an integrating sphere attachment, with BaSO4 as a reflectance standard. X-ray photoelectron spectroscopy (XPS) was conducted by a Thermo ESCALAB 250 with Al Kα (1486.6 eV) line at 150 W. Field emission scanning electron microscopy (SEM) images were obtained using an FEI Sirion200 scanning electron microscope operated at 5.00 kV and 10 mA (USA). Energy dispersive X-ray spectroscopy (EDS) was obtained on an FEI Sirion200 scanning electron microscope, using a scanning voltage of 5.00 kV (USA). The microstructure and crystallinity of the samples were analyzed by transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM) and selected area electron diffraction (SAED) on a JEOL-2011 transmission electron microscope with an accelerating voltage of 200 kV (Japan). Inductively coupled plasma optical emission spectrometry (ICP-OES) was carried out on a Varian 720 ICP OES spectrophotometer. A NOVA 2000e (Quantachrome Instruments, USA) instrument was used to measure the Brunauer–Emmett–Teller (BET) surface areas of the samples at liquid nitrogen temperature (77.3 K).2.3. Photocatalytic activity evaluation
The photocatalytic activities of the as-prepared samples were evaluated by degrading MO and phenol solution. A 500 W Xe lamp coupled with a UV cutoff filter (λ > 400 nm) was used as the light source. In a typical experiment, 0.10 g photocatalyst was first dispersed in 50 mL of MO or phenol solution (10 mg L−1) and then magnetically stirred in the dark for 30 min to establish an adsorption–desorption equilibrium between the photocatalyst and the MO or phenol solution. During visible light irradiation, about 2.6 mL of the suspension was withdrawn at given time intervals, centrifuged and filtered through a 0.22 μm Millipore filter to remove the photocatalyst particles. Finally, the absorbance intensity of the MO solution was measured using a 722s spectrophotometer at 464 nm with deionized water as a reference sample, while the absorption of the phenol solution was analyzed by a TU-1901 UV-vis spectrophotometer.2.4. Photoelectric investigation
Photocurrents of the samples were investigated on an electrochemical workstation (CHI660E, Chenhua Instruments Co. Shanghai, China) in a standard three-electrode configuration with a working electrode, a counter electrode (a platinum wire) and a reference electrode (Ag/AgCl, 3.0 M KCl). The working electrode was processed as follows: 1.0 mL chitosan dispersion with 0.05 g photocatalyst was sonicated for 20 min to produce a homogeneous slurry. Then, the slurry was coated on the conductive surface of indium-tin oxide (ITO) glass to form a uniform film (1.00 cm2) and sintered in air at 80 °C for 2 h. Phosphate buffered saline (PBS) aqueous solution including 0.1 M Na2HPO4 and 0.1 M NaH2PO4 was employed as the electrolyte. A 500 W Xe lamp with a 400 nm cutoff filter was utilized to provide the necessary visible light.Surface photovoltage (SPV) spectroscopy was measured on an instrument made by us. Monochromatic light was obtained by passing light from a 500 W Xe lamp (LSH-X500) through a double prism monochromator (Omni-λ 3005). The slit width of the entrance and exit is 1 mm. A lock-in amplifier (SR830-DSP), synchronized with a light chopper (SR540), was employed to amplify the photovoltage signal. The range of the modulating frequency is from 20 to 70 Hz. The spectral resolution is set as 1 nm.
3. Results and discussion
3.1. Photocatalytic activity of I-BiOBr samples
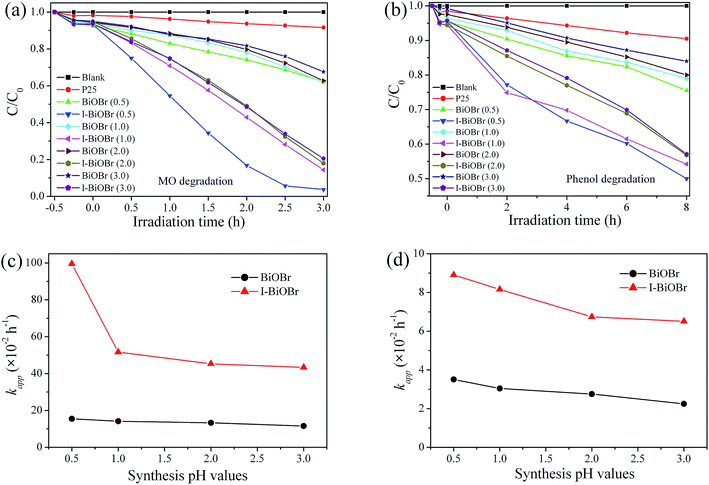
The photocatalytic activities of the I-BiOBr samples prepared at different pH values (0.5–3.0) were investigated by degrading MO and phenol under visible light (λ > 400 nm). Fig. 1a shows that all the I-BiOBr samples exhibited higher MO removal efficiency than the corresponding pure BiOBr. Similar activity variation for the degradation of colorless phenol over I-BiOBr was also observed (Fig. 1b). Thus, the activity of BiOBr could be largely enhanced via I− ion doping in the process of contaminants removal. To reveal the relationship between the pH values during synthesis and the activity of I-BiOBr, the apparent pseudo-first-order rate constants (kapp) calculated on the basis of an apparent pseudo-first-order kinetics equation37 are presented in Fig. 1c and d. It is interesting to note that, with the decrease in pH values from 3.0 to 0.5, the kapp values for BiOBr slowly increased, whereas those of I-BiOBr increased distinctly. When the pH value was decreased to 0.5, the I-BiOBr (0.5) sample displayed the best activity for MO removal (96.0%, kapp = 1.00 h−1) and phenol degradation (51.0%, kapp = 0.09 h−1). Moreover, the photocatalytic activities of the I-BiOBr samples prepared at pH values from 4.0 to 6.0 are shown in Fig. S1 (see the ESI†). The significant pH effects on the activity of I-BiOBr toward contaminant degradation compelled us to study the changes in its morphology, composition, structure, light absorption and I− ions doping amount with the pH variation.3.2. Characterization
In addition, the band gap energies of the as-prepared samples were further estimated using eqn (3)45,46 based on the abovementioned DRS results:
| αhν = A(hν − Eg)n/2 | (3) |
 | ||
| Fig. 5 SEM images of (a) BiOBr (0.5), (b) BiOBr (1.0), (c) BiOBr (2.0), (d) BiOBr (3.0), (e) I-BiOBr (0.5), (f) I-BiOBr (1.0), (g) I-BiOBr (2.0) and (h) I-BiOBr (3.0). | ||
To obtain information about the dispersion of every element in the I-BiOBr sample, I-BiOBr (0.5) was selected to conduct the EDS mapping of elements (Fig. 6). It should be noted that Bi, O, Br and I elements were uniformly distributed throughout the I-BiOBr (0.5) sample. This indicates that I− was homogeneously doped in the I-BiOBr (0.5) sample. The corresponding EDS spectra of the samples are further provided in Fig. 7. They demonstrate that the pure BiOBr samples are composed of Bi, O and Br elements, while I-BiOBr samples contain Bi, O, Br and I elements. On the basis of the EDS spectra, the contents of I element in I-BiOBr samples were calculated to be 3.18 at%, 3.01 at%, 2.83 at% and 2.52 at% as the pH increased from 0.5 to 3.0. Because the EDS results just reflect the surface element content of the photocatalysts, the whole element contents in the I-BiOBr samples were further measured by ICP technology. The contents of I− ions were 2.64 at%, 2.42 at%, 1.49 at% and 2.24 at% in I-BiOBr (0.5), I-BiOBr (1.0), I-BiOBr (2.0) and I-BiOBr (3.0) samples. The doping amount of I− ions was located in a narrow range and was not consistent with the activity changes of I-BiOBr with increasing pH values. This suggests that the doping amount of I− ions was not the main factor for determining the activity differences of I-BiOBr samples.
3.3. Activity enhancement mechanism
Based on the abovementioned analysis, it can be summarized that I-BiOBr could be endowed with excellent visible light activity by I− ions doping, which is also distinctly affected by the synthesis pH values. Thus, it is necessary to thoroughly understand the activity enhancement mechanism of I-BiOBr under visible light.Generally, the activity of a semiconductor photocatalyst is mainly determined by the electron–hole separation efficiency, which can be well reflected by the photoelectrochemical properties. Thus, the photocurrent signals of BiOBr and I-BiOBr were measured under visible light (λ > 400 nm). Fig. 10a shows that all the samples displayed positive photocurrent signals with different intensities during the visible light irradiation. Concretely speaking, I-BiOBr had higher photocurrent intensity than the corresponding BiOBr. In addition, photocurrent change tendencies of the samples completely coincided with their activity variations. Thus, the enhanced photocurrent intensity indicates strengthened electron–hole separation efficiency and improved activity of I-BiOBr. In addition, the different synthesis pH values induced variable electron–hole separation efficiency and photocatalytic activity in I-BiOBr. The photocurrent of the I-BiOBr samples prepared at pH values from 4.0 to 6.0 are also supplied in Fig. S2a (see the ESI†).
 | ||
| Fig. 10 (a) Transient photocurrent of the samples under visible light (λ > 400 nm) and (b) surface photovoltage properties of all the samples. | ||
It is well known that surface photovoltage (SPV) arises whenever light-induced excess charge carriers are separated in space. Therefore, the formation of a SPV signal is determined by the fundamental properties of light absorption and the transport of excess carriers in a semiconductor material.55 Fig. 10b presents the SPV spectra of all the samples. For pure BiOBr, only one positive SPV signal in the range of 300–420 nm corresponded to the band-to-band transition of BiOBr, which reveals that electrons were transferred to bulk and holes were transferred to surface.56 This is a representative feature of an n-type semiconductor in SPV, where the positive hole of a surface space charge area migrated to the surface.57,58 Furthermore, the SPV peak intensity decreased from BiOBr (3.0) to BiOBr (0.5). This can be understood through analyzing the number change of the surface positive charge. In Fig. 11, the zeta potentials of all the BiOBr samples were negative, suggesting that BiOBr preferentially adsorbed more negative Br− ions originating from the dissociation of BiOBr in water (eqn (4)). The surface adsorbed Br− ions on BiOBr can grasp holes to form active Br0, which can directly oxidize the MO and phenol.59,60 Because the content of adsorbed Br− ions decreased from BiOBr (0.5) to BiOBr (3.0), the consumption of surface holes would have gradually decreased. Consequently, the number of residual surface holes increased and resulted in the enhancement of SPV signals.
| BiOBr → BiO+ + Br− | (4) |
In contrast, there were two evident positive SPV peaks in the I-BiOBr samples. In addition to the similar peak at about 300–420 nm originating from the band-to-band transition of BiOBr, a sub-band transition of I-BiOBr in the range of 420–570 nm resulted from the transition of I− ion impurity levels. The SPV signal range was consistent with the DRS results. The doped I− ion significantly decreased the intrinsic SPV signal of BiOBr but generated a new sub-band transition. On the basis of a consideration similar to BiOBr, the decreased SPV signal of I-BiOBr was mainly caused by the counteraction of surface positive holes trapped by adsorbed Br− ions. The zeta potentials of all the I-BiOBr were more negative than the corresponding BiOBr samples, which will cause more Br− ions to be adsorbed on the surface of I-BiOBr and produce much lower SPV signals. The SPV spectra of the I-BiOBr samples prepared at pH values from 4.0 to 6.0 are also supplied in Fig. S2b (see the ESI†). Thus, variation in the synthesis pH was considered a facile and efficient way to modulate the photoelectric properties of BiOBr and I-BiOBr samples.
The stability of a photocatalyst is crucial in view of practical applications. For this reason, the I-BiOBr (0.5) sample was tested 5 times for MO degradation. As seen in Fig. 12, I-BiOBr (0.5) retained its excellent photocatalytic activity after 5 consecutive photocatalytic experiments under visible light (λ > 400 nm). This good photocatalytic activity and acceptable stability imply that I-BiOBr (0.5) could be a promising candidate for practical applications.
According to the reported method,14 the CB and VB potentials of BiOBr were calculated to be 0.29 and 3.07 eV. Furthermore, because BiOBr is an n-type semiconductor, the energy band structure of I-BiOBr mainly depends on the VB potential change in comparison with that of BiOBr. Thus, the VB potentials of I-BiOBr were estimated to be 2.34–2.37 eV from BiOBr (0.5) to BiOBr (3.0), as shown in Fig. 13. Under the visible light (λ > 400 nm), photoinduced electrons were excited and transferred from VB to CB of I-BiOBr. Then, the adsorbed O2 captured the electrons and generated the reactive ˙O2−, which could directly decompose MO and phenol.37 Simultaneously, the VB holes immediately migrated to the surface and reacted with Br− to form reactive Br0, which also immediately oxidized MO and phenol. Thus the adsorbed surface Br− ions play an important role in modulating the number of surface holes, which led to the different activity changes of I-BiOBr samples that were due to the increase in synthesis pH values.
 | ||
| Fig. 13 Schematic illustration of the effects of pH on the energy band structure and photocatalytic activities of I-BiOBr. | ||
4. Conclusions
In summary, a series of I-BiOBr samples have been controllably fabricated by regulating the synthesis pH values of the reaction solution. The lower pH values resulted in higher visible light activity of I-BiOBr for the elimination of methyl orange and phenol. The different surface charge states mainly determined the activity change differences of I-BiOBr samples owing to the similar doping amount of I− ions at various synthesis pH values. This study has shed light on the activity modulation of ion doped BiOX photocatalysts through utilizing the effects of synthesis pH.Acknowledgements
This study was financially supported by the Natural Science Foundation of China (51472005, 51172086, and 51272081) and the Natural Science Foundation of Educational Committee of Anhui Province (KJ2014A221, KJ2015A027, and gxyqZD2016413).References
- Z. X. Li, Y. Shen, Y. H. Guan, Y. H. Hu, Y. H. Lin and C. W. Nan, J. Mater. Chem. A, 2014, 2, 1967–1973 CAS.
- X. G. Sun, Y. Y. Zhang, C. M. Li, Z. F. Zhang, Z. Peng, H. Y. Si, J. M. Zhang and Y. T. Li, J. Alloys Compd., 2015, 638, 254–260 CrossRef CAS.
- J. Shang, W. C. Hao, X. J. Lv, T. M. Wang, X. L. Wang, Y. Du, S. X. Dou, T. F. Xie, D. J. Wang and J. O. Wang, ACS Catal., 2014, 4, 954–961 CrossRef CAS.
- L. Y. Ding, R. J. Wei, H. Chen, J. C. Hu and J. L. Li, Appl. Catal., B, 2015, 172–173, 91–99 CAS.
- Y. C. Yang, Y. Liu, J. H. Wei, C. X. Pan, R. Xiong and J. Shi, RSC Adv., 2014, 4, 31941–31947 RSC.
- H. Irie, Y. Watanabe and K. Hashimoto, J. Phys. Chem. B, 2003, 107, 5483–5486 CrossRef CAS.
- J. H. Park, S. W. Kim and A. J. Bard, Nano Lett., 2006, 6, 24–28 CrossRef CAS PubMed.
- L. J. Zhang, S. Li, B. K. Liu, D. J. Wang and T. F. Xie, ACS Catal., 2014, 4, 3724–3729 CrossRef CAS.
- M. Luo, Y. Liu, J. C. Hu, H. Liu and J. J. Li, ACS Appl. Mater. Interfaces, 2012, 4, 1813–1821 CAS.
- X. Zhang, Z. H. Ai, F. L. Jia and L. Z. Zhang, J. Phys. Chem. C, 2008, 112, 747–753 CAS.
- C. J. Huang, J. L. Hu, S. Cong, Z. G. Zhao and X. Q. Qiu, Appl. Catal., B, 2015, 174–175, 105–112 CrossRef CAS.
- S. M. Wang, Y. Guan, L. P. Wang, W. Zhao, H. He, J. Xiao, S. G. Yang and C. Sun, Appl. Catal., B, 2015, 168–169, 448–457 CrossRef CAS.
- X. J. Wang, W. Y. Yang, F. T. Li, J. Zhao, R. H. Liu, S. J. Liu and B. Li, J. Hazard. Mater., 2015, 292, 126–136 CrossRef CAS PubMed.
- J. Cao, B. Y. Xu, H. L. Lin, B. D. Luo and S. F. Chen, Chem. Eng. J., 2012, 185–186, 91–99 CrossRef CAS.
- T. J. Yan, X. Y. Yan, R. R. Guo, W. N. Zhang, W. J. Li and J. M. You, Catal. Commun., 2013, 42, 30–34 CrossRef CAS.
- J. Wang, C. Dong, B. B. Jiang, K. L. Wu, J. Sun, X. Z. Li, W. J. Zhang, B. Zhang and X. W. Wei, Mater. Lett., 2014, 131, 108–111 CrossRef CAS.
- Z. C. Yang, J. Li, F. X. Cheng, Z. Chen and X. P. Dong, J. Alloys Compd., 2015, 634, 215–222 CrossRef CAS.
- J. B. Sun, J. R. Song, M. A. Gondal, S. Shi, Z. X. Lu, Q. Y. Xu, X. F. Chang, D. H. Xiang and K. Shen, Res. Chem. Intermed., 2015, 41, 6941–6955 CrossRef CAS.
- C. L. Yu, C. F. Fan, X. J. Meng, K. Yang, F. F. Cao and X. Li, React. Kinet., Mech. Catal., 2011, 103, 141–151 CrossRef CAS.
- X. L. Li, X. M. Mao, X. C. Zhang, Y. F. Wang, Y. W. Wang, H. Zhang, X. G. Hao and C. M. Fan, Sci. China, Ser. B, 2015, 58, 457–466 CrossRef CAS.
- C. L. Yu, F. F. Cao, G. Li, R. F. Wei, J. C. Yu, R. C. Jin, Q. Z. Fan and C. Y. Wang, Sep. Purif. Technol., 2013, 120, 110–122 CrossRef CAS.
- X. Zhang and L. Z. Zhang, J. Phys. Chem. C, 2010, 114, 18198–18206 CAS.
- G. H. Jiang, X. H. Wang, Z. Wei, X. Li, X. G. Xi, R. B. Hu, B. L. Tang, R. J. Wang, S. Wang, T. Wang and W. X. Chen, J. Mater. Chem. A, 2013, 1, 2406–2410 CAS.
- G. H. Jiang, R. J. Wang, X. H. Wang, X. G. Xi, R. B. Hu, Y. Zhou, S. Wang, T. Wang and W. X. Chen, ACS Appl. Mater. Interfaces, 2012, 4, 4440–4441 CAS.
- Z. S. Liu, B. T. Wu, Y. L. Zhao, J. N. Niu and Y. B. Zhu, Ceram. Interfaces, 2014, 40, 5597–5603 CrossRef CAS.
- Z. F. Jia, F. M. Wang, F. Xin and B. Q. Zhang, Ind. Eng. Chem. Res., 2011, 50, 6688–6694 CrossRef CAS.
- A. Dash, S. Sarkar, V. N. K. B. Adusumalli and V. Mahalingam, Langmuir, 2014, 30, 1401–1409 CrossRef CAS PubMed.
- G. H. Jiang, X. Li, Z. Wei, T. T. Jiang, X. X. Du and W. X. Chen, Powder Technol., 2014, 260, 84–89 CrossRef CAS.
- G. H. Jiang, X. Li, Z. Wei, X. H. Wang, T. T. Jiang, X. X. Du and W. X. Chen, Powder Technol., 2014, 261, 170–175 CrossRef CAS.
- M. Q. He, W. B. Li, J. X. Xia, L. Xu, J. Di, H. Xu, S. Yin, H. M. Li and M. N. Li, Appl. Surf. Sci., 2015, 331, 170–178 CrossRef CAS.
- K. Zhang, D. Q. Zhang, J. Liu, K. X. Ren, H. Luo, Y. J. Peng, G. S. Li and X. B. Yu, CrystEngComm, 2012, 14, 700–707 RSC.
- B. Zhang, G. B. Ji, Y. S. Liu, M. A. Gondal and X. F. Chang, Catal. Commun., 2013, 36, 25–30 CrossRef CAS.
- H. L. Lin, X. Li, J. Cao, S. F. Chen and Y. Chen, Catal. Commun., 2014, 49, 87–91 CrossRef CAS.
- G. Q. Tan, L. L. Zhang, H. J. Ren, S. S. Wei, J. Huang and A. Xia, ACS Appl. Mater. Interfaces, 2013, 5, 5186–5193 CAS.
- D. W. Chu, Y. Masuda, T. Ohji and K. Kato, Langmuir, 2010, 26, 2811–2815 CrossRef CAS PubMed.
- N. M. Kinsinger, A. Dudchenko, A. Wong and D. Kisailus, ACS Appl. Mater. Interfaces, 2013, 5, 6247–6254 CAS.
- F. T. Li, Y. Zhao, Y. J. Hao, X. J. Wang, R. H. Liu, D. S. Zhao and D. M. Chen, J. Hazard. Mater., 2012, 239–240, 118–127 CrossRef CAS PubMed.
- J. Zhang, S. W. Liu, J. G. Yu and M. Jaroniec, J. Mater. Chem., 2011, 21, 14655–14662 RSC.
- S. H. Shen, L. Zhao, Z. H. Zhou and L. J. Guo, J. Phys. Chem. C, 2008, 112, 16148–16155 CAS.
- X. Xiao, R. P. Hu, C. Liu, C. L. Xing, X. X. Zuo, J. M. Nan and L. S. Wang, Chem. Eng. J., 2013, 225, 790–797 CrossRef CAS.
- J. Cao, X. Li, H. L. Lin, B. Y. Xu, B. D. Luo and S. F. Chen, Mater. Lett., 2012, 76, 181–183 CrossRef CAS.
- X. Y. Xiao, J. Jiang and L. Z. Zhang, Appl. Catal., B, 2013, 142–143, 487–493 CrossRef CAS.
- X. M. Jia, J. Cao, H. L. Lin, Y. Chen, W. F. Fu and S. F. Chen, J. Mol. Catal. A: Chem., 2015, 409, 94–101 CrossRef CAS.
- S. Bouhadoun, C. Guillard, F. Dapozze, S. Singh, D. Amans, J. Bouclé and N. H. Boime, Appl. Catal., B, 2015, 174–175, 367–375 CrossRef CAS.
- J. Cao, X. Li, H. L. Lin, S. F. Chen and X. L. Fu, J. Hazard. Mater., 2012, 239–240, 316–324 CrossRef CAS PubMed.
- J. Zeng, H. Wang, Y. C. Zhang, M. K. Zhu and H. Yang, J. Phys. Chem. C, 2007, 111, 11879–11887 CAS.
- J. Cao, B. Y. Xu, B. D. Luo, H. L. Lin and S. F. Chen, Catal. Commun., 2011, 13, 63–68 CrossRef CAS.
- K. Li, H. B. Zhang, Y. P. Tang, D. W. Ying, Y. L. Xu, Y. L. Wang and J. P. Jia, Appl. Catal., B, 2015, 164, 82–91 CrossRef CAS.
- J. Cao, B. Y. Xu, H. L. Lin and S. F. Chen, Chem. Eng. J., 2013, 228, 482–488 CrossRef CAS.
- H. Liu, W. R. Cao, Y. Su, Y. Wang and X. H. Wang, Appl. Catal., B, 2012, 111–112, 271–279 CrossRef CAS.
- J. J. Hu, G. Q. Xu, J. W. Wang, J. Lv, X. Y. Zhang, Z. X. Zheng, T. Xie and Y. C. Wu, New J. Chem., 2014, 38, 4913–4921 RSC.
- Z. H. Ai, W. K. Ho and S. C. Lee, J. Phys. Chem. C, 2011, 115, 25330–25337 CAS.
- L. Kong, Z. Jiang, H. H. Lai, R. J. Nicholls, T. C. Xiao, M. O. Jones and P. P. Edwards, J. Catal., 2012, 293, 116–125 CrossRef CAS.
- Y. N. Huo, J. Zhang, M. Miao and Y. Jin, Appl. Catal., B, 2012, 111–112, 334–341 CrossRef CAS.
- I. Mora-sero, T. Dittrich, A. Belaidi, G. Crarcia-belmonte and J. Bisquert, J. Phys. Chem. B, 2005, 109, 14932–14938 CrossRef CAS PubMed.
- L. Kronik and Y. Shapira, Surf. Sci. Rep., 1999, 37, 1–206 CrossRef CAS.
- V. Timoshenko, V. Duzhko and T. Dittrich, Phys. Status Solidi A, 2000, 182, 227 CrossRef CAS.
- L. J. Zhang, R. Zheng, S. Li, B. K. Liu, D. J. Wang, L. L. Wang and T. F. Xie, ACS Appl. Mater. Interfaces, 2014, 6, 13406–13412 CAS.
- J. Cao, B. D. Luo, H. L. Lin and S. F. Chen, J. Mol. Catal. A: Chem., 2011, 344, 138–144 CrossRef CAS.
- P. Wang, B. B. Huang, X. Y. Zhang, X. Y. Qin, H. Jin, Y. Dai, Z. Y. Wang, J. Y. Wei, J. Zhan, S. Y. Wang, J. P. Wang and M. H. Wangbo, Chem.–Eur. J., 2009, 15, 1821–1824 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra22943j |
| This journal is © The Royal Society of Chemistry 2016 |