A binuclear Cu(i) complex as a novel catalyst towards the direct synthesis of N-2-aryl-substituted-1,2,3-triazoles from chalcones†
Abstract
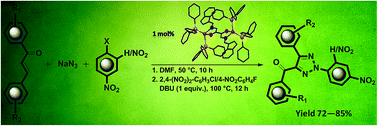
A binuclear Cu(I) complex containing a N′,N′-bis{(1H-indol-3-yl)methylene}oxalohydrazide (H2bioh) ligand has been synthesized and characterized. The molecular structures of the synthesized compounds have been determined by single crystal X-ray diffraction. The crystal structures are stabilized by inter- and intra-molecular π–π stacking and C–H⋯π interactions. The Cu(I)-complex has successfully been employed as an efficient catalyst for one-pot operation involving the azide–chalcone click reaction and subsequent arylation has been developed. The complex exhibits excellent catalytic activity with significantly low catalyst loading. The overall process is insensitive to air and moisture and can be manipulated under ambient temperature with operational ease.

 Please wait while we load your content...
Please wait while we load your content...