Novel caffeine derivatives with antiproliferative activity†
Martin Andrsab,
Darina Muthnac,
Martina Rezacovac,
Martina Seifrtovac,
Pavel Simanc,
Jan Korabecnyab,
Ondrej Benekab,
Rafael Dolezalb,
Ondrej Soukupab,
Daniel Junab and
Kamil Kuca*b
aDepartment of Toxicology and Military Pharmacy, Faculty of Military Health Sciences, University of Defence, Trebesska 1575, 500 01 Hradec Kralove, Czech Republic
bBiomedical Research Centre, University Hospital Hradec Kralove, Sokolska 581, 500 05 Hradec Kralove, Czech Republic. E-mail: kamil.kuca@fnhk.cz; Tel: +420 495 832 923
cDepartment of Medical Biochemistry, Faculty of Medicine in Hradec Kralove, Charles University in Prague, Simkova 870, 500 38 Hradec Kralove, Czech Republic
First published on 24th March 2016
Abstract
Caffeine is probably the best known and most widely used psychoactive substance in the world. Beside its psychoactive effects, caffeine has been found to affect the cell cycle and DNA repair, as a consequence of the inhibition of ATM and ATR kinases. These two DNA damage response kinases, members of the phosphatidylinositol 3-kinase related protein kinase family, represent very attractive anticancer drug targets. Their inhibition can selectively sensitize cancer cells to DNA damaging agents and even kill various tumour cells in monotherapy. We developed a series of caffeine derivatives and evaluated their antiproliferative effects on 11 human tumour cell lines and compared them against caffeine and a standard ATR inhibitor VE-821. Although the new caffeine derivatives did not achieve the overall potency of VE-821, several compounds exhibited enhanced antiproliferative activity compared to caffeine and in some cell lines showed at least comparable activity to VE-821.
Introduction
Caffeine is a naturally occurring CNS stimulant and one of the most frequently consumed psychoactive substances in the world. Apart from its well-known actions on the neural and cardiovascular systems, caffeine also exhibits notable effects on cell cycle function and regulation, apoptosis and DNA repair.1–3 Caffeine-prevented G2 arrest and imperfect DNA repair before the S phase were observed more than 30 years ago, when it was found that caffeine increases the lethality of various cytotoxic agents such as nitrogen mustard.4,5 Since then caffeine has been the subject of many studies, which revealed the inhibition of ataxia telangiectasia mutated (ATM), ataxia telangiectasia and Rad3-related (ATR) kinases as the major cause of these effects.6,7ATM and ATR are atypical serine/threonine protein kinases, which belong to the phosphatidylinositol 3-kinase (PI3K) related protein kinase (PIKK) family. They play a major role in the complex signal network of transduction pathways of DNA damage responses (DDR).8,9 ATM kinase is one of the main regulators in response to DNA double strand breaks (DSBs), hyperthermia, reactive oxygen species or replication stress. DSBs are the most dangerous type of DNA damage, and their repair is necessary for cell viability or correct cell division. They are caused by ionizing radiation (IR), various cancer chemotherapeutics or by mechanical stress on chromosomes. The naming of ATM kinase originated from the rare multisystem disorder ataxia telangiectasia, which is caused by mutation in the ATM gene. As well as cerebellar ataxia and telangiectasia, patients suffer from neurodegeneration, cancer predisposition and extreme sensitivity to DNA damage due to genomic instability and cell cycle anomalies.10 ATR is, on the other hand, mainly a replication stress kinase and is responsible for proper DNA replication and reparation of single strand breaks (SSB). ATR gene mutation is embryonically lethal, possibly due to imperfect DNA replication in the absence of ATR.11
In current cancer research, inhibition of ATM or ATR is desirable, because it provides an opportunity to enhance the conventional genotoxic cancer therapy (i.e., radiotherapy, chemotherapy). Not only can it increase the effectiveness of the treatment but it also enables specific targeting of tumour cells, which are often more sensitive to DDR intervention.12,13
The earliest inhibitor of PI3K and PIKK to be discovered was a fungal metabolite wortmannin (Fig. 1). Wortmannin showed a very strong potency against almost all PI3K members. However, low selectivity, irreversible inhibition and high toxicity of this compound prevented its further use.14,15 The other naturally occurring inhibitor is the aforementioned caffeine. Methylxanthines in general are known to exert effects on the cell cycle and DNA repair, through the mechanism of inhibition of ATM and ATR kinases. The inhibition potency of caffeine is very weak (IC50 value for ATM and ATR is 200 and 1100 μM, respectively), and hence cannot itself be considered as a potential radiosensitizer, due to its numerous other effects on the human body. The concentration in blood needed for the desired effect would be associated with fatal tachyarrhythmias.12,16
The attractiveness of ATR and ATM kinases is reflected in the efforts of several pharmaceutical companies and academic institutions for the development of new small molecule inhibitors for these kinases.16,17 Currently, the most advanced ATM inhibitor is KU-60019 (Fig. 1), which exhibited strong and safe radio- and chemosensitization of tumour cells and suppression of cell proliferation and migration.18 Among the ATR inhibitors, VE-822 (VX-970)19,20 and AZD6738![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21,22 are already in phase I of clinical trials for assessing safety and pharmacokinetics, both when administered alone and in combination with cytotoxic drugs. These compounds proved to be very effective in selective targeting of ATM or p53 deficient tumour cells, while they showed no severe toxicity on normal tissue.13,19
21,22 are already in phase I of clinical trials for assessing safety and pharmacokinetics, both when administered alone and in combination with cytotoxic drugs. These compounds proved to be very effective in selective targeting of ATM or p53 deficient tumour cells, while they showed no severe toxicity on normal tissue.13,19
Despite the extensive research devoted to these kinases, to date only a few promising inhibitors have been disclosed. The reason for the paucity of selective and potent inhibitors can be found in the absence of high-resolution information about the kinase domain structures of these kinases and in the high similarity between the members of PI3K and PIKK families, making the development of novel inhibitors an unmet goal.23
In this work, we turned our efforts to caffeine and investigated the potency of several novel caffeine derivatives on the proliferation of 11 tumour cell lines, plausibly to find compounds with a highlighted antiproliferative effect caused by ATM or ATR inhibition. As a starting compound we used theophylline, to which were attached various non-polar aromatic or aliphatic substituents at position 7- via a methylene linker (i.e., benzyls, methylcyclohexyl). The selection of initial four substituents followed the Topliss scheme for mapping the structure–activity relationships.24 The resulting series of 17 compounds were biologically evaluated; however, their poor aqueous solubility limited further biological testing. Hence, we tried to improve the physicochemical properties and possibly the activity by polar substituent attachments via an amide linker to positions 4- and 3- of the benzyl moiety, similarly as was applied in KU-60019.18 Unfortunately, this structural modification resulted in derivatives endowed with very low to no activity. Overall, a few of the prepared caffeine derivatives affected the proliferation of various tumour cell lines, and in some cell lines they exhibited at least comparable activity to that of the standard ATR inhibitor VE-821 (Fig. 1).
Results and discussion
Chemistry
The general synthetic pathway for the novel caffeine derivatives is displayed in Scheme 1 and described in ESI.† The initial series of 7-substituted caffeine derivatives (1–17) was obtained in good-to-excellent yields (62–92%) by reaction of theophylline with differently substituted benzyl bromides, 4-methylcyclohexyl bromide or (1-bromoethyl)benzene in the presence of potassium carbonate in DMF.25 The two nitrophenyl derivatives (15, 16) were then quantitatively reduced by zinc in acetic acid to the corresponding amino derivatives (18, 19).26 These 4- and 3-amino derivatives (18, 19) were used as starting compounds for preparation of a second series of derivatives with enhanced solubility. The acylation of 18 and 19 with chloroacetyl chloride afforded intermediates 20 and 26, which were subsequently treated with different secondary amines to afford the final compounds 21–25 and 27 with overall yields ranging from 25 to 37%.All synthesized compounds (1–27, except intermediates 20, 26) were sufficiently characterized by 1H NMR, 13C NMR and high-resolution mass spectrometry (HRMS) analyses. According to LC with UV detection (λ = 254 nm; see ESI†) the uncalibrated purity of the compounds was >98%.
Cell proliferation after caffeine derivative treatment
Single dose testing of growth inhibition on the screening panel of 11 human tumour cell lines was performed with 25 different caffeine derivatives at a concentration of 20 μM. At the end of the incubation period (48 hours), cell proliferation was determined by WST-1 proliferation assay and related to the proliferation of untreated control cells. The one-dose data of all the screened compounds are in ESI Fig. S1, Table S2.†Amongst the caffeine derivatives tested, compound 6 showed the highest overall activity (Fig. 2, S1; Table 1, S2†) as it caused a drop of proliferation to below 50% of the control in 4 cell lines (below 75% of control in 8 lines) from 11 lines tested, and its growth percentage (GP) value was 63 with the range from 19 to 108. Based on the GP values, derivatives 12 and 16 followed with GP values 67 (range 26–91) and 69 (range 32–84), respectively. As a positive control, we used a selective inhibitor of ATR, VE-821. Treatment with this inhibitor at a concentration of 10 μM led to at least 50% proliferation decrease in 6 cell lines tested (10 lines below 75%) and its GP value was 52 (range 33–82). As expected, the parent compound of the series, caffeine at concentration 20 μM exhibited almost no activity on cells' proliferation (GP 97, range 83–120).
| Compound | R | Mean GP | Range of GP | The most sensitive cell lines |
|---|---|---|---|---|
| a Structure displayed in Scheme 1.b Substituents refer to R′ in Scheme 1. | ||||
| 1 | H | 93 | 78–133 | HeLa, A2780, PANC-1 |
| 2 | 4-MeO | 84 | 51–106 | A2780, Jurkat, HeLa |
| 3 | 4-Me | 83 | 41–103 | A2780, Jurkat, HeLa |
| 4 | 4-Cl | 92 | 57–116 | A2780, Jurkat, HeLa |
| 5 | 3,5-diMe | 78 | 26–105 | A2780, Jurkat, HeLa |
| 6 | 4-iPr | 63 | 19–108 | A2780, MOLT-4, AGS |
| 7 | 2-Me | 81 | 42–98 | A2780, Jurkat, COLO-201 |
| 8 | 4-CN | 95 | 60–132 | A2780, HeLa, COLO-201 |
| 9 | 4-Br | 98 | 49–117 | A2780, AGS, Jurkat |
| 10 | 4-COOMe | 81 | 43–106 | A2780, HeLa, HT-29 |
| 11 | 4-CF3 | 78 | 46–96 | A2780, MOLT-4, Jurkat |
| 12 | 4-MeS | 67 | 26–91 | A2780, MOLT-4, HeLa |
| 13 | 3-Br | 73 | 34–112 | A2780, MOLT-4, HeLa |
| 14 | a | 88 | 73–112 | HeLa, SW-480, PANC-1 |
| 15 | 4-NO2 | 95 | 68–127 | A2780, HeLa, Jurkat |
| 16 | 3-NO2 | 69 | 32–84 | A2780, Jurkat, HeLa |
| 17 | a | 96 | 76–130 | COLO-201, HT-29, Jurkat |
| 18 | 4-NH2 | 83 | 62–103 | HT-29, HeLa, SW-480 |
| 19 | 3-NH2 | 86 | 65–104 | A2780, COLO-201, Jurkat |
| 21 | N-Methylpiperazineb | 96 | 81–122 | COLO-201, HT-29, PANC-1 |
| 22 | Morpholineb | 87 | 70–106 | COLO-201, A2780, Jurkat |
| 23 | Piperidineb | 88 | 75–98 | A2780, MOLT-4, Jurkat |
| 24 | Diethylamineb | 96 | 88–103 | MCF-7, MOLT-4, A549 |
| 25 | Diethanolamineb | 103 | 93–124 | A549, AGS, MCF-7 |
| 27 | a | 101 | 73–120 | A2780, Jurkat, MOLT-4 |
| VE-821 | — | 52 | 33–82 | COLO-201, HeLa, A2780 |
| Caffeine | — | 97 | 83–120 | SW-480, AGS. Jurkat |
Focussing on the cell types, A2780 (ovarian carcinoma) seemed to be the most sensitive cell line to the action of caffeine derivatives, as its proliferation dropped below 50% after treatment with 11 derivatives from a total of 25. GP value for A2780 was calculated as the mean of the percentage growth after each derivative treatment, and reached 61 with range from 26 to 103 (Fig. 3). On the other hand, the proliferation of MCF-7, A549, PANC-1 and SW-480 cell lines was almost unaffected by any of the prepared derivatives, including caffeine. Low activity (proliferation above 60%) was also observed after incubation with VE-821.
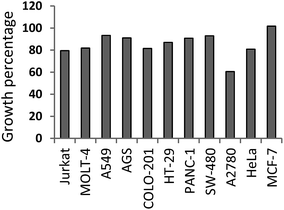 | ||
| Fig. 3 Overall sensitivity of tumour cell lines to caffeine derivatives. GP was calculated for each cell line as mean value of percentage expression of proliferation after caffeine derivative treatment (1–27). The order of cell lines in this figure corresponds with numbers in Fig. 2. | ||
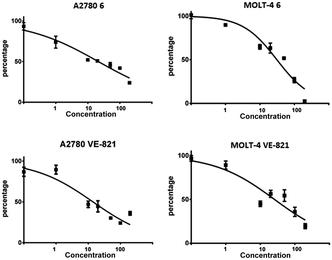
For two of the most sensitive cell lines, A2780 and MOLT-4, the cytotoxic dose dependency of compound 6 was evaluated and compared to VE-821. The dose response curves for compound 6 and VE-821 in A2780 ovarian cancer cell line and MOLT-4 leukemic cell line after 48 h are shown in Fig. 4. Half maximal inhibitory concentration (IC50) was calculated from the measured results (Table S1†). The caffeine derivative 6 affected the A2780 and MOLT-4 cells with IC50 values of 21 and 31 μM, respectively; and VE-821 with 14 and 26 μM, respectively.
To confirm that the derivative 6 acts as an inhibitor of ATR or ATM kinase we evaluated its effect on radiation-induced serine 345 phosphorylation of Chk1 (catalysed mainly by ATR) and threonine 68 phosphorylation of Chk2 (catalysed mainly by ATM).27–29 HL-60 cells were treated with the standard ATR inhibitor VE-821 or caffeine derivative 6 and irradiated by the dose of 6 Gy. The irradiation induces DNA damage, triggers DDR and results in significant increase in phosphorylated Chk1 and Chk2. The serine 345 phosphorylation of Chk1 was inhibited by standard ATR inhibitor VE-821 as well as by compound 6 in all tested concentrations (Fig. 5). As we expected, the ATR kinase inhibitor VE-821 did not prevent threonine 68 phosphorylation of Chk2. The changes in Chk2 phosphorylation in presence of derivatives 6 were not significant. From these data, we can assume that derivative 6 is a potent inhibitor of ATR.
Whilst caffeine was inefficient at a concentration of 20 μM, several other derivatives bearing non-polar substitution at position 7- exhibited a moderate antiproliferative effect on various tumour cell lines. Introduction of a benzyl moiety itself (1) brought no activity improvement compared to caffeine; however, the activity was noticeably affected when the benzyl moiety contained small functional groups. From the GP values of the synthesized compounds we cannot deduce any direct dependence on different substituents on the aromatic ring. The most effective were compounds 6 and 12, which both have a hydrophobic (isopropyl, methylthio) at position 4- of phenyl, whereas the derivatives with an electron-withdrawing substituent at this position (–Cl, –Br, –CN, –NO2) were inactive. From this data we can assume that aliphatic substituents in this position enhance the antiproliferative activity. However, the third ranked most active compound 16 stands out somewhat as it bears the nitro group at position 3- of the benzyl. Experiments probing the effect of saturation of the non-polar part of the molecule in compound 14 seemed to indicate a negligible effect on activity compared to its aromatic counterpart 1. Similarly, the introduction of a chiral centre to explore the effect of rotation of the aromatic ring relative to the core purine in 17 did not reveal any fluctuation in activity, possibly due to no topological preference in the binding site.
In order to model partitioning of compounds 6 and 12, we carried out a chromatographic analysis with Immobilized Artificial Membrane (IAM) column which mimic the lipid environment in cell membranes. Capacity factors proved that 6 (k = 6.36) interacts strongly with IAM column, whilst 12 (k = 2.85) exhibits significantly weaker retention. Comparing to 1 (k = 0.99), both compounds 6 and 12 are more hydrophobic, which may partially elucidate the observed enhancement of the biological activities by improved penetration to the cells.
The second series was prepared with the aim of increasing the solubility as well as to investigate the effects of polar substitution on activity. Disappointingly, although the 4- and 3-substituted derivatives (20–25, 27) had slightly better solubility, their antiproliferative activity was low to nil (GP values of 88–103). Thus apparently only non-polar substituents are suitable for modification of the benzyl ring.
The results showed that beneficial modification of the benzyl moiety of the caffeine derivatives is very limited, and larger, polar and electron withdrawing groups all led to the ablation of activity. Therefore, investigation of modifications in the xanthine core will be the next step in seeking to uncover the plausible potential of novel caffeine hybrids.
Although the overall effects of the newly synthesized derivatives on cell proliferation are not very significant, certain amelioration compared to the standards is noteworthy. Compound 6 showed pronounced ATR inhibition and affected some cell lines (i.e. MOLT-4, A2780 or HeLa) in a manner similar to VE-821.
Conclusions
In this work, we prepared 25 novel caffeine derivatives through simple modification and examined their effect on the proliferation towards 11 tumour cell lines, which could result from ATR kinase inhibition. Caffeine itself was not active at the given concentration, but several new derivatives (6, 12 and 16) exhibited similar, although not as strong, activities as the potent ATR inhibitor VE-821. Unfortunately, the active derivatives suffered from low aqueous solubility, which limits their biological evaluation. Efforts to increase the solubility and antiproliferative activity by modification of the benzyl moiety were not successful, leaving the xanthine core as the next target for modification.Acknowledgements
This work was supported by the specific research (SV/FVZ201402), by Long Term Development plan – 1011, by MH CZ – DRO (University Hospital Hradec Kralove, No. 00179906) and the PRVOUK P37/01 programme initiated by Charles University in Prague. The authors are grateful to Ian McColl MD, PhD for assistance with the manuscript.Notes and references
- B. B. Fredholm, K. Bättig, J. Holmén, A. Nehlig and E. E. Zvartau, Pharmacol. Rev., 1999, 51, 83–133 CAS.
- D. Robertson, J. Frolich, R. Carr, J. Watson, J. Hollifield, D. Shand and J. Oates, N. Engl. J. Med., 1978, 298, 181–186 CrossRef CAS PubMed.
- A. M. Bode and Z. Dong, Cancer Lett., 2007, 247, 26–39 CrossRef CAS PubMed.
- Y. Fujiwara and T. Kondo, Biochem. Biophys. Res. Commun., 1972, 47, 557–564 CrossRef CAS PubMed.
- C. C. Lau and A. B. Pardee, Proc. Natl. Acad. Sci. U. S. A., 1982, 79, 2942–2946 CrossRef CAS.
- J. N. Sarkaria, E. C. Busby, R. S. Tibbetts, P. Roos, Y. Taya, L. M. Karnitz and R. T. Abraham, Cancer Res., 1999, 59, 4375–4382 CAS.
- B. B. S. Zhou, P. Chaturvedi, K. Spring, S. P. Scott, R. A. Johanson, R. Mishra, M. R. Mattern, J. D. Winkler and K. K. Khanna, J. Biol. Chem., 2000, 275, 10342–10348 CrossRef CAS PubMed.
- Y. Shiloh, Nat. Rev. Cancer, 2003, 3, 155–168 CrossRef CAS PubMed.
- Y. Shiloh, Curr. Opin. Genet. Dev., 2001, 11, 71–77 CrossRef CAS PubMed.
- M. F. Lavin, Nat. Rev. Mol. Cell Biol., 2008, 9, 759–769 CrossRef CAS PubMed.
- K. A. Cimprich and D. Cortez, Nat. Rev. Mol. Cell Biol., 2008, 9, 616–627 CrossRef CAS PubMed.
- J. N. Sarkaria and J. S. Eshleman, Semin. Radiat. Oncol., 2001, 11, 316–327 CrossRef CAS PubMed.
- P. M. Reaper, M. R. Griffiths, J. M. Long, J.-D. Charrier, S. Maccormick, P. A. Charlton, J. M. C. Golec and J. R. Pollard, Nat. Chem. Biol., 2011, 7, 428–430 CrossRef CAS PubMed.
- J. N. Sarkaria, R. S. Tibbetts, E. C. Busby, A. P. Kennedy, D. E. Hill and R. T. Abraham, Cancer Res., 1998, 58, 4375–4382 CAS.
- M. P. Wymann, G. Bulgarelli-Leva, M. J. Zvelebil, L. Pirola, B. Vanhaesebroeck, M. D. Waterfield and G. Panayotou, Mol. Cell. Biol., 1996, 16, 1722–1733 CrossRef CAS PubMed.
- M. Andrs, J. Korabecny, E. Nepovimova, D. Jun, Z. Hodny, S. Moravcova and K. Kuca, Mini-Rev. Med. Chem., 2014, 14, 805–811 CrossRef CAS PubMed.
- M. Andrs, J. Korabecny, E. Nepovimova, D. Jun, Z. Hodny and K. Kuca, Curr. Cancer Drug Targets, 2016, 16, 200–208 CrossRef CAS PubMed.
- S. E. Golding, E. Rosenberg, N. Valerie, I. Hussaini, M. Frigerio, X. F. Cockcroft, W. Y. Chong, M. Hummersone, L. Rigoreau, K. A. Menear, M. J. O'Connor, L. F. Povirk, T. van Meter and K. Valerie, Mol. Cancer Ther., 2009, 8, 2894–2902 CrossRef CAS PubMed.
- E. Fokas, R. Prevo, J. R. Pollard, P. M. Reaper, P. A. Charlton, B. Cornelissen, K. A. Vallis, E. M. Hammond, M. M. Olcina, W. Gillies McKenna, R. J. Muschel and T. B. Brunner, Cell Death Dis., 2012, 3, e441 CrossRef CAS PubMed.
- R. Prevo, E. Fokas, P. M. Reaper, P. A. Charlton, J. R. Pollard, W. G. McKenna, R. J. Muschel and T. B. Brunner, Cancer Biol. Ther., 2012, 13, 1072–1081 CrossRef CAS PubMed.
- C. D. Jones, K. Blades, K. M. Foote, S. M. Guichard, P. J. Jewsbury, T. McGuire, J. W. Nissink, R. Odedra, K. Tam, P. Thommes, P. Turner, G. Wilkinson, C. Wood and J. W. Yates, Cancer Res., 2013, 73, 2348 Search PubMed.
- K. M. Foote, K. Blades, A. Cronin, S. Fillery, S. S. Guichard, L. Hassall, I. Hickson, X. Jacq, P. J. Jewsbury, T. M. McGuire, J. W. M. Nissink, R. Odedra, K. Page, P. Perkins, A. Suleman, K. Tam, P. Thommes, R. Broadhurst and C. Wood, J. Med. Chem., 2013, 56, 2125–2138 CrossRef CAS PubMed.
- M. Andrs, J. Korabecny, D. Jun, Z. Hodny, J. Bartek and K. Kuca, J. Med. Chem., 2015, 58, 41–71 CrossRef CAS PubMed.
- J. G. Topliss, J. Med. Chem., 1977, 20, 463–469 CrossRef CAS PubMed.
- J. W. Daly, W. L. Padgett and M. T. Shamim, J. Med. Chem., 1986, 29, 1305–1308 CrossRef CAS PubMed.
- C. Cano, K. Saravanan, C. Bailey, J. Bardos, N. J. Curtin, M. Frigerio, B. T. Golding, I. R. Hardcastle, M. G. Hummersone, K. A. Menear, D. R. Newell, C. J. Richardson, K. Shea, G. C. M. Smith, P. Thommes, A. Ting and R. J. Griffin, J. Med. Chem., 2013, 56, 6386–6401 CrossRef CAS PubMed.
- J. M. Wagner and S. H. Kaufmann, Pharmaceuticals, 2010, 3, 1311–1334 CrossRef CAS.
- J. Bartek and J. Lukas, Cancer Cell, 2003, 3, 421–429 CrossRef CAS PubMed.
- J. Vavrova, L. Zarybnicka, E. Lukasova, M. Rezacova, E. Novotna, Z. Sinkorova, A. Tichy, J. Pejchal and K. Durisova, Radiat. Environ. Biophys., 2013, 52, 471–479 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra22889a |
| This journal is © The Royal Society of Chemistry 2016 |