Aging behavior of the silicone dielectric elastomers in a simulated marine environment†
Abstract
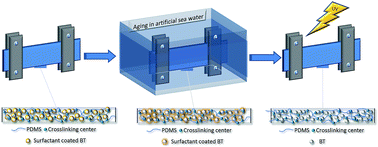
A series of silicone–barium titanate composites (multiple specimens of each sample), designed as dielectric elastomeric films to be used as active elements in wave energy conversion devices, were immersed in artificial sea water in pseudo-dynamic conditions. While some of specimens were extracted after half a year and subsequently subjected to UV irradiation for 500 h (ASW1/2 + UV procedure), the rest were kept in the saline environment for one year (ASW procedure). The changes that occurred in the structure and morphology as well as in mechanical and dielectric properties were assessed by comparing the obtained results to those of the original samples. Thus, the surface and cross-section morphology was studied by Scanning Electron Microscopy (SEM) having an attached Energy Dispersive X-ray system (EDX), which was used for qualitative elemental analysis and elemental mapping. Changes in surface roughness due to the aging of samples were estimated on the basis of Atomic Force Microscopy (AFM) measurements. The thermal transitions were identified from the Differential Scanning Calorimetry (DSC) data and based on these, the crystallinity degree of the samples was evaluated in each of the three stages. The changes in the structural order were also verified by Wide Angle X-ray Diffraction (WAXD). The tensile toughness, as the amount of energy per volume unit that the material can absorb until failure, was estimated by the area under tensile stress–strain curves. The toughness at 100% elongation was determined on the basis of cyclic stress–strain curves of the original samples as an indirect measure of the energy that the elastic material could release when force that acted on it is removed, like in an energy harvesting system. The aging effect on the viscoelasticity of the samples was evaluated by dynamic mechanical analysis (DMTA), while dielectric spectroscopy was used to estimate the changes in dielectric properties.

 Please wait while we load your content...
Please wait while we load your content...