Enhanced nucleation of polypropylene by metal–organic frameworks (MOFs) based on aluminium dicarboxylates: influence of structural features†
Mohan Raj Maniab,
Ramesh Chellaswamy*ab,
Yogesh N. Maratheab and
Vijayamohanan K. Pillaibc
aPolymer Science and Engineering Division, Polymers and Advanced Materials Laboratory, CSIR-National Chemical Laboratory, Dr. Homi Bhabha Road, Pune-411 008, India. E-mail: c.ramesh@ncl.res.in
bAcademy of Scientific and Innovative Research (AcSIR), New Delhi 110 025, India
cCSIR-Central Electrochemical Research Institute, Karaikudi-630006, India
First published on 21st December 2015
Abstract
Metal–organic frameworks (MOFs) based on aluminium dicarboxylates provide a new platform for the enhanced nucleation of isotactic polypropylene (iPP). For instance, aluminium dicarboxylates exhibit a unique butterfly-like structure similar to that of carboxylate-alumoxanes and correlates well with the nucleation characteristics of iPP. A subtle change in the structure of the ligand backbone (fumarate/succinate) does not alter the framework structure despite changing the hydrophilic/hydrophobic character and its subsequent nucleation characteristics. This suggests that the nucleating agent should facilitate favourable interaction with hydrophobic iPP for efficient nucleation. Further, a systematic variation of the alkyl chain length in the Al-dicarboxylate does not change the nucleation efficiency considerably, even though it increases the distance between the octahedral alumina chains in the metal–organic framework, suggesting that the butterfly-like structure present in the framework is a key aspect for nucleation. Finally, the significance of the orientational conformation of the dicarboxylate around the metal centre for the nucleation is confirmed by the poor nucleation efficiency of chromium and zirconium suberate MOFs where the orientation of suberate would be different from that of aluminium suberate due to the difference in the ligation of the carboxylate group. The present work thus provides valuable pathways for developing new nucleating agents based on MOFs with appropriate selection and orientation of the organic linkers around the metal centre.
Introduction
Control of the crystallization of isotactic polypropylene (iPP) is extremely important as it regulates the processing speed, energy spent for processing, aesthetics of the processed polymer etc., polypropylene crystallizes during cooling from the melt via a homogeneous nucleation mechanism. However, the formation of nuclei of critical size is a kinetically controlled process and needs a high degree of supercooling. The productivity can be increased by decreasing the supercooling temperature and also by allowing the material to solidify at a higher temperature. A foreign material, called a nucleating agent in a minute amount, is usually added during the polymer processing to increase the crystallization temperature. This is mainly because the addition of nucleating agents is expected to reduce the free energy barrier of nucleation by providing a suitable surface for iPP to interact and crystallize at higher temperature.2–4 The nucleating materials morphology in the polymer melt or the nucleation mechanism are very difficult to establish by experimental techniques because the concentration of the additive is in very small amounts. Nevertheless, Senker and co-workers5 used solid state NMR spectroscopy to understand the nucleating materials morphology in the polymer matrix.Nucleation effect of iPP can be determined from the increase in crystallization temperature compared to the unnucleated iPP. Although a vast number of compounds has been shown to nucleate iPP,6–8 only few are commercially successful, which includes sodium benzoate,9 sorbitol derivatives,10–12 1,3,5-benzenetrisamide derivatives13,14 and phenylene phosphate derivatives.15,16 Polyvinyl cyclohexane17 and carboxylate-alumoxanes1 have also been shown to be efficient nucleating agents for isotactic polypropylene. This structural diversity of the known nucleating agents pose major problems in finding new nucleating agents based on chemical structures and functionalities. Nevertheless, the molecular structure of dibenzylidene sorbitol (DBS),10 sodium 2,2′-methylene-bis-(4,6-di-t-butylphenylene)phosphate18 and carboxylate-alumoxanes1 shows a butterfly-like or v-shaped conformation and surprisingly all are highly efficient in nucleating iPP. As far as carboxylate-alumoxane is concerned, the butterfly-like structure originated from the bridging coordination of COO− and oxo ligand towards Al centres1,19 is of critical importance. Recently, it has been reported that the aluminium fumarate (Al-FumA) metal–organic framework (MOF) is also built with the similar linkages20 which also shows a butterfly-like structure. More importantly, this butterfly structure forms a linear channel on the surface and runs along the a-axis.
Herein, we report, for the first time, MOFs based on aluminium dicarboxylates as effective nucleating agents for iPP along with possible mechanistic explanation for the nucleation. Also, we show that a minor structural variation (unsaturated to saturated ligand backbone) has profound influence in the nucleation of iPP. Further, the effect of framework structure in nucleation by changing, (i) the backbone length of the dicarboxylic acid (varying number of intervening CH2 groups) and (ii) the metal centre (Al, Cr and Zr) with the same dicarboxylate linker are also discussed in order to provide better insights into the selection of generic nucleating agents.
Experimental
Materials
Aliphatic dicarboxylic acids were procured from Sigma-Aldrich. Metal nitrates were purchased form Vijay chemicals Ltd, India. Isotactic polypropylene (iPP) homopolymer in the form of powder was kindly supplied by Reliance Industries Ltd., Mumbai. The weight-average molecular weight (Mw) obtained by gel permeation chromatography (GPC) in 1,2,4-trichlorobenzene at 160 °C and found to be 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. The polydispersity index (Mw/Mn) is 4.6.
000. The polydispersity index (Mw/Mn) is 4.6.
Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. Cr(NO3)3·9H2O and ZrO(NO3)2·xH2O salts were used to prepare Cr-suberate and Zr-suberate compounds respectively.
10. Cr(NO3)3·9H2O and ZrO(NO3)2·xH2O salts were used to prepare Cr-suberate and Zr-suberate compounds respectively.The NAs were premixed with iPP powder and extruded at 200 °C using a DSM twin-screw micro extruder. Samples were allowed to mix for 2 min in a barrel at 100 rpm screw speed before the extrusion. The crystallization temperature of the nucleated iPP was measured by Differential Scanning Calorimetry (model TA Q100) in N2 atmosphere at a flow rate of 50 ml min−1. About 4–6 mg of sample was heated to 200 °C and held for 2 min. The melt crystallization temperature, TC, was the peak crystallization temperature on subsequent cooling at 10 °C min−1. The sample was removed from the pan after cooling to room temperature and used in WAXS experiments using a Rigaku Micromax-007HF diffractometer operating at 40 kV and 30 mA. FT-IR spectra with a resolution of 2 cm−1 were collected using a Perkin-Elmer spectrometer (model Spectrum GX) with samples in KBr pellets. Thermogravimetric analyses (TGA) were performed using Perkin-Elmer simultaneous thermal analyzer (model STA 6000) under nitrogen atmosphere at a heating rate of 10 °C min−1. The nitrogen gas sorption–desorption experiments (0–1 bar) were performed using a Quantachrome Quadrasorb automatic volumetric instrument. Brunauer–Emmett–Teller (BET) method was used to measure the surface area of the samples.
Results and discussion
The nucleation efficiency (NE) of aluminium dicarboxylates on iPP along with crystallization temperature (TC) are given in Table 1 and the corresponding differential scanning calorimetry (DSC) thermograms are shown in Fig. S1 (ESI†). The TC is measured on cooling the polymer melt at 10 °C min−1. It is to be mentioned that the higher TC indicates higher nucleation efficiency and higher crystallization rate. The nucleation efficiency1,21 studies show that all aluminium dicarboxylates are effective nucleating agents and increase the TC by ca. 12 to 17 °C which is obviously higher than that of unnucleated iPP.| S. No | Compound (NAs)b | –(CH2)n– | TC (°C) | NEc (%) |
|---|---|---|---|---|
| a n – number of intervening CH2 groups in the dicarboxylate linker.b Concentration of NAs: 2000 ppm.c NE calculated following the method given by Fillon et al.1,21 | ||||
| 1 | Pristine iPP | — | 113.0 | 0 |
| 2 | Self-nucleated iPP | — | 140.2 | 100 |
| 3 | DMDBS | — | 130.5 | 64 |
| 4 | Al-fumarate (unsaturated) | 2 | 118.0 | 18 |
| 5 | Al-succinate (saturated) | 2 | 128.6 | 56 |
| 6 | Al-glutarate (Al-GluA) | 3 | 129.6 | 61 |
| 7 | Al-adipate (Al-AdiA) | 4 | 127.0 | 51 |
| 8 | Al-pimelate (Al-PimA) | 5 | 124.5 | 41 |
| 9 | Al-suberate (Al-SubA) | 6 | 130.0 | 62 |
| 10 | Al-azelate (Al-AzeA) | 7 | 118.5 | 20 |
| 11 | Al-sebacate (Al-SebA) | 8 | 125.7 | 45 |
| 12 | Al-dodecanedioate (Al-DDA) | 10 | 127.5 | 52 |
| 13 | Cr-suberate (Cr-SubA) | 6 | 120.6 | 28 |
| 14 | Zr-suberate (Zr-SubA) | 6 | 119.6 | 24 |
The dependence of crystallization temperature (TC) on the concentration of aluminium suberate (Al-SubA) is shown in Fig. 1 along with similar data for the standard nucleating agent 1,3:2,4-bis(3,4-dimethylbenzylidene)sorbitol (DMDBS) for comparison. Fig. 1 clearly shows that the 2000 ppm of Al-SubA is as efficient as DMDBS at equal concentration. Interestingly, Al-SubA is efficient even at 3 ppm level and increases TC by ca. 10 °C higher than unnucleated iPP. On the other hand, DMDBS shows nucleation effect for concentrations only above 1000 ppm. In fact, Fig. 1 appears to be similar to the figure of dependence of crystallization temperature on the concentration of p-tert-butylbenzoate (PTBBA)-alumoxane published elsewhere.1 The higher nucleation efficiency of Al-SubA at extremely low concentration indicates that Al-dicarboxylates are highly dispersive in the iPP matrix during the melt mixing process in the extruder. The WAXS patterns of the unnucleated and nucleated iPP are shown in Fig. S2 (ESI†). The Al-dicarboxylates reported here nucleates iPP in the α phase and is independent of the length of the dicarboxylates. It is worth pointing out that the calcium dicarboxylates can nucleate iPP either in the α phase or in the β phase.22
 | ||
| Fig. 1 Variation of concentration of nucleating agents (NAs) with the crystallization temperature (TC) of isotactic polypropylene (iPP). | ||
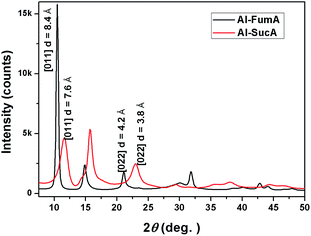
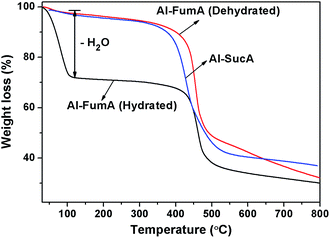
The structure of Al-dicarboxylate derived from Al-fumarate (Al-FumA) has been solved recently and shown to be a MOF.20 Fumaric and succinic acids have the same number of intervening carbon atoms but with and without unsaturated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond and the respective molecular structures are shown in Fig. 2. Hence, it is expected that the succinic acid would form a similar MOF structure with aluminium as that of Al-FumA. The WAXS patterns of the Al-FumA and Al-SucA are shown in Fig. 3. The X-ray pattern of Al-SucA is comparable with that of Al-FumA with a systematic shift in the peak positions towards the larger 2θ values. For instance, the d spacing of 011 plane in Al-FumA is 8.4 Å while the same distance in Al-SucA is measured to be 7.6 Å and both the compounds show 022 plane at d[011]/2 distance. The decrease in the d spacing is expected as succinate is more flexible due to C–C single bond as compared to the rigid fumarate.20 The flexibility is further confirmed by TGA (Fig. 4) which shows that Al-SucA degrades at a lower temperature than dehydrated Al-FumA. In addition, FTIR spectrum (Fig. S3†) of Al-SucA shows asymmetric (νas) COO− stretching vibrations at 1610 cm−1 and symmetric (νs) COO− vibrations at 1457 cm−1, which are characteristics of bridging coordination.20,23 The bridging hydroxyl group vibrations ν(μ2-OH), typical for corner-sharing AlO4(OH)2 octahedra is observed at 3693 cm−1.20,24 Hence, it can be concluded that the Al-SucA possess metal–organic framework (MOF) structure very similar to Al-FumA in which trans-corner sharing octahedral AlO4(OH)2 chains are connected by succinate linkers as shown in Fig. 5. Furthermore, nitrogen sorption–desorption isotherm studies (Fig. S4 and S5, ESI†) show BET surface area to be ca. 144 m2 g−1 and 300 m2 g−1 for Al-SucA and Al-FumA respectively. Lower surface area of Al-SucA indicates less porosity than the Al-FumA. This can be explained on the basis of the incorporation of protruding hydrogen atoms of the succinate linker (sp3 hybridized methylene groups) towards the pore walls.
C double bond and the respective molecular structures are shown in Fig. 2. Hence, it is expected that the succinic acid would form a similar MOF structure with aluminium as that of Al-FumA. The WAXS patterns of the Al-FumA and Al-SucA are shown in Fig. 3. The X-ray pattern of Al-SucA is comparable with that of Al-FumA with a systematic shift in the peak positions towards the larger 2θ values. For instance, the d spacing of 011 plane in Al-FumA is 8.4 Å while the same distance in Al-SucA is measured to be 7.6 Å and both the compounds show 022 plane at d[011]/2 distance. The decrease in the d spacing is expected as succinate is more flexible due to C–C single bond as compared to the rigid fumarate.20 The flexibility is further confirmed by TGA (Fig. 4) which shows that Al-SucA degrades at a lower temperature than dehydrated Al-FumA. In addition, FTIR spectrum (Fig. S3†) of Al-SucA shows asymmetric (νas) COO− stretching vibrations at 1610 cm−1 and symmetric (νs) COO− vibrations at 1457 cm−1, which are characteristics of bridging coordination.20,23 The bridging hydroxyl group vibrations ν(μ2-OH), typical for corner-sharing AlO4(OH)2 octahedra is observed at 3693 cm−1.20,24 Hence, it can be concluded that the Al-SucA possess metal–organic framework (MOF) structure very similar to Al-FumA in which trans-corner sharing octahedral AlO4(OH)2 chains are connected by succinate linkers as shown in Fig. 5. Furthermore, nitrogen sorption–desorption isotherm studies (Fig. S4 and S5, ESI†) show BET surface area to be ca. 144 m2 g−1 and 300 m2 g−1 for Al-SucA and Al-FumA respectively. Lower surface area of Al-SucA indicates less porosity than the Al-FumA. This can be explained on the basis of the incorporation of protruding hydrogen atoms of the succinate linker (sp3 hybridized methylene groups) towards the pore walls.
 | ||
| Fig. 5 A schematic representation of Al-SucA and Al-FumA (MOFs) structures with possible interaction of iPP with the channel wall of the butterfly-like structure. | ||
The MOFs based on Al-dicarboxylates show butterfly-like structures due to their coordination linkages around Al centre, as shown in Fig. 5 and is similar to carboxylate-alumoxanes structure discussed elsewhere.1 More specifically, the Al-dicarboxylate MOF structures can form a linear channel on the surface along the a-axis in 3D structure as clearly seen in the Al-FumA MOF structure.20 By analogy, it can be concluded that all the dicarboxylates based on aluminium do have a MOF structure. This is also supported by the WAXS patterns (Fig. 6a) which indicates that all Al-dicarboxylate MOFs are highly crystalline and show a sharp peak in the range of (2θ) 5°–12°, arising from the reflection of 011 plane representing dicarboxylate direction.20 The d[011] spacing increases with increasing number of intervening methylene groups as shown in Fig. 6b; with observable odd–even effect.
Interestingly, Al-dicarboxylate MOFs like carboxylate-alumoxanes, act as an efficient nucleating agents for iPP. As with carboxylate-alumoxanes,1 the interaction of iPP molecular segment with the butterfly-like structure may be responsible for the efficient nucleation of Al-dicarboxylate MOFs as shown in Fig. 5. Nevertheless, the Al-dicarboxylate MOFs and carboxylate-alumoxanes differ from each other due to the mode of interaction with iPP. For instance, carboxylate-alumoxane provides aromatic cleft for the iPP interaction1 while the higher nucleation efficiency of Al-dicarboxylate MOFs is attributed to the interaction of iPP helical chain with alkyl group of the butterfly-like channel. The repeat distance along the channel, which is along the a-axis is 6.8 Å (ref. 20) and is very close to the 6.5 Å of the iPP 31 helical chain length suggesting lattice matching between nucleating agent and iPP crystal. However, the Al-FumA and Al-AzeA are exceptions and do not nucleate iPP even though the butterfly-like channel is present along the a-direction and no change in the a-axis repeat unit. It is interesting to compare the structure and nucleation behaviour of Al-FumA (unsaturated Al-dicarboxylate) and Al-SucA (saturated Al-dicarboxylate). While both Al-FumA and Al-SucA have very similar structures, the difference in the nucleation behaviour may be explained due to the difference in the interaction with iPP. More specifically, the Al-FumA is hydrophilic in nature as evident from TGA and is shown in Fig. 4. Hydrated Al-FumA shows a weight loss due to absorbed water molecules which is in agreement with the literature,20 whereas Al-SucA and the higher analogous saturated Al-dicarboxylates does not absorb water molecules even after prolonged treatment with water as seen from Fig. 4 and S6 (ESI†) respectively are indicating the hydrophobicity of these materials. Further, it may be noted that the cavity in the structures of higher analogous saturated Al-dicarboxylates will be larger than that of the cavity of Al-FumA. In the case of Al-FumA, the hydrophilicity arises due to bridged hydroxyl and carboxylate oxygens which interact with water molecules through hydrogen bonding as reported by Alvarez et al.20 The hydrophilic sites are open because of the backbone structure of fumarate where the H atoms (sp2 hybridized CH groups) are coplanar with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond and hence do not hinder the water molecules interacting with them. However, in the case of Al-SucA, the hydrophilic sites are shielded by the protruding hydrogens of the succinate linker (sp3 hybridized CH2 groups) and impart hydrophobic character to the channel wall. Hence, it can be concluded that the subtle change in the structure of ligand backbone (fumarate to succinate) brings remarkable variation in the hydrophilic/hydrophobic character of the channel determined by the butterfly-like structure and subsequent interactions to effect nucleation characteristics of iPP. Therefore, poor nucleation of Al-FumA is due to hydrophilic nature, perhaps caused by the repulsive interactions with hydrophobic iPP while enhanced nucleation of Al-SucA is due to hydrophobic channel which can facilitate attractive interaction with hydrophobic iPP. This suggests that the butterfly structure is prerequisite but favourable interaction is also essential for the effective nucleation of iPP.
C double bond and hence do not hinder the water molecules interacting with them. However, in the case of Al-SucA, the hydrophilic sites are shielded by the protruding hydrogens of the succinate linker (sp3 hybridized CH2 groups) and impart hydrophobic character to the channel wall. Hence, it can be concluded that the subtle change in the structure of ligand backbone (fumarate to succinate) brings remarkable variation in the hydrophilic/hydrophobic character of the channel determined by the butterfly-like structure and subsequent interactions to effect nucleation characteristics of iPP. Therefore, poor nucleation of Al-FumA is due to hydrophilic nature, perhaps caused by the repulsive interactions with hydrophobic iPP while enhanced nucleation of Al-SucA is due to hydrophobic channel which can facilitate attractive interaction with hydrophobic iPP. This suggests that the butterfly structure is prerequisite but favourable interaction is also essential for the effective nucleation of iPP.
Nucleation efficiency studies (Table 1) show that all Al-dicarboxylates are effective nucleating agents although they have different d[011] spacing (Fig. 6b). This indicates that the increase in the intervening methylene groups simply increases the length of the wing of the butterfly-like cavity (Fig. 5) while the a-axis remain unaltered and hence does not affect the nucleation efficiency considerably. It is worth pointing out that the common feature of efficient nucleating agents presented in this work and published in literature1,10,18 necessitate a butterfly-like or v-shaped conformation of the single unit and the linear channel arising out of it on the surface in 3D structure. These channels with favourable attraction can align iPP molecular segment in the channel to initiate the nucleation process.
As mentioned earlier, the Al-azelate shows poor nucleation although the X-ray pattern indicates that the structure of Al-AzeA is analogous to other Al-dicarboxylates. At present there is no explanation for its poor nucleation behaviour although it may be speculated that the azelate ligand can bend and coordinate with the same octahedral alumina chain sporadically and this possibility is restricted in other dicarboxylates due to chain length. Hence, it can be expected that sporadic bending of azelate chains could mask the butterfly-like cavity consequently leading to poor interaction and poor nucleation of iPP. Al-dicarboxylates with 9 and higher odd number intervening methylene groups may exhibit similar behavior but needs to be verified experimentally.
High nucleation efficiency of Al-dicarboxylates is accomplished by the presence of butterfly-like structure which arises mainly due to the carboxylate and hydroxo bridging coordination towards Al centres. The chelation binding is restricted in alumina owing to the ring strain.23 Hence, it is possible to alter the butterfly-like structure by choosing the metal centre like chromium (Cr), zirconium (Zr) etc. Which can coordinate with carboxylate group via a chelation mode along with bridging and unidentate binding.25,26 Further, the Zr can accommodate up to eight ligands in its coordination sphere due to larger size.27 To clarify the above aspect, the MOFs based on Al-suberate (Al-SubA), Cr-suberate (Cr-SubA) and Zr-suberate (Zr-SubA) were prepared followed by an analysis of their nucleation properties. The nature of coordination mode of carboxylate towards metal centre can be identified by FTIR spectroscopy.28,29 A comparison of the FTIR spectra of Al-SubA, Cr-SubA and Zr-SubA is shown in Fig. 7, which reveals the symmetric (νs) COO− stretching at the same position in all the above three compounds. However, the position of the asymmetric (νas) COO− stretching changes with respect to its associated metal centre. As discussed earlier, the COO− group is bound to Al centre via a bridging coordination. However, the COO− group in the Zr-SubA is involved in chelation binding mode as characterized by a lower Δ value of 92 cm−1 (νas − νs of COO−) as compared to a Δ value of 140 cm−1 for bridging carboxylate with aluminium. This chelation binding is further confirmed by the presence of peak a at 478 cm−1 corresponding to Zr–O stretching in the chelated metal carboxylate ring.26 On the other hand, the Cr-SubA complex shows both bridging and chelated COO− coordination as revealed by the presence of two peaks (1613 cm−1 and 1541 cm−1) for asymmetric (νas) COO− stretching vibrations. The difference in the ligation of carboxylate group towards Zr and Cr strongly suggests that the orientation of suberate ligands around them would be different from that of Al-SubA and consequently, forming a different MOF structure.
WAXS pattern of Al-SubA and Cr-SubA shown in Fig. S7 (ESI†) indicates that both Al-SubA and Cr-SubA have similar crystalline order and the difference in the diffraction profiles suggest that their framework structures are different. Zr-SubA does not show any diffraction peaks indicating amorphous nature of MOF. TGA (Fig. S8, ESI†) shows all the three MOFs to have thermal stability above 300 °C, much above the iPP processing temperature. Surprisingly, Zr-SubA and Cr-SubA show poor nucleation efficiency although the suberate is very efficient in nucleating iPP when it forms MOF structure in combination with aluminium. This indicates that the suberate alone is not responsible for the effective nucleation of iPP emphasizing the critical role played by the orientational conformation of the ligand around the metal centre for the iPP nucleation, which is in complete agreement with our previous work on carboxylate-alumoxanes.1
Conclusions
The present study reveals that the nucleating agent should provide a channel (formed by the butterfly-like structure in the present as well as in the most of the other NAs) that can facilitate favourable interactions with iPP for enhanced nucleation. The variation of the alkyl chain length between the alumina chains in the MOF does not alter the nucleation efficiency considerably, suggesting that the butterfly-like structure present in the metal–organic framework holds the key for controlling nucleation. Further, the poor nucleation of Cr-SubA and Zr-SubA as compared to Al-SubA corroborates the critical role played by the orientational conformation of the dicarboxylate in the nucleation of iPP. Although, more exhaustive study is needed to understand the mechanism of nucleation in a quantitative manner, the present work undoubtedly provides valuable pathways for developing new nucleating agents based on the MOFs with the proper selection and orientation of the organic linkers around the metal centre. This study also demonstrates that physical properties of the materials can be enhanced multi fold if the interaction occurs in the molecular scale.Acknowledgements
The project was funded by Department of Science and Technology (DST) New Delhi, vide no SR/S3/CE/070/2009. M. Mani acknowledges CSIR, New Delhi for the Senior Research Fellowship.Notes and references
- M. Mani, R. Chellaswamy, Y. N. Marathe and V. Pillai, Chem. Commun., 2015, 51, 10026 RSC.
- G. C. Oppenlander, Science, 1968, 159, 1311 CAS.
- H. Zweifel, Plastics Additives Handbook, 5th edn, Hanser Publications, Munich, 2001, p. 952 Search PubMed.
- J. Mercier, Polym. Eng. Sci., 1990, 30, 270 CAS.
- M. Schmidt, J. Wittmann, R. Kress, H.-W. Schmidt and J. Senker, Chem. Commun., 2012, 49, 267–269 RSC.
- M. Walker, A. Smith and A. Karim, Langmuir, 2003, 19, 6582 CrossRef CAS.
- T. Bauer, R. Thomann and R. Mülhaupt, Macromolecules, 1998, 31, 7651 CrossRef CAS.
- R. S. Vaaben, A. Aguilar, F. Avalos and L. F. R. Valle, J. Therm. Anal. Calorim., 2008, 93(3), 947 CrossRef.
- (a) H. N. Beck, J. Appl. Polym. Sci., 1967, 11, 673 CrossRef CAS; (b) G.-S. Jang, W.-J. Cho and C.-S. Ha, J. Polym. Sci., Part B: Polym. Phys., 2001, 39, 1001 CrossRef CAS.
- T. Smith, D. Masilamani, L. Bui, Y. Khanna, R. Bray, W. Hammond, S. Curran, J. Belles and S. Binder-Castelli, Macromolecules, 1994, 27, 3147 CrossRef CAS.
- T. Smith, D. Masilamani, L. Bui, R. Brambilla, Y. Khanna and K. Garbriel, J. Appl. Polym. Sci., 1994, 52, 591 CrossRef CAS.
- M. Kristiansen, M. Werner, T. Tervoort, P. Smith, M. Blomenhofer and H.-W. Schmidt, Macromolecules, 2003, 36, 5150 CrossRef CAS.
- M. Blomenhofer, S. Ganzleben, D. Hanft, H.-W. Schmidt, M. Kristiansen, P. Smith, K. Stoll, D. Mäder and K. Hoffmann, Macromolecules, 2005, 38, 3688 CrossRef CAS.
- F. Abraham, R. Kress, P. Smith and H.-W. Schmidt, Macromol. Chem. Phys., 2013, 214, 17 CrossRef CAS.
- S. Yoshimoto, T. Ueda, K. Yamanaka, A. Kawaguchi, E. Tobita and T. Haruna, Polymer, 2001, 42, 9627 CrossRef CAS.
- Y. Shi and Z. Xin, J. Appl. Polym. Sci., 2012, 125, 2963 CrossRef CAS.
- A. Menyhárd, M. Gahleitner, J. Varga, K. Bernreitner, P. Jääskeläinen, H. Øysæd and B. Pukánszky, Eur. Polym. J., 2009, 45, 3138 CrossRef.
- G. Zhang, Z. Xin, J. Yu, Q. Gui and S. Wang, J. Macromol. Sci., Part B: Phys., 2003, 42, B42 Search PubMed , (3 & 4) 467.
- L. Kalita, R. Pothiraja, V. Saraf, M. Walawalkar, R. Butcher and R. Murugavel, J. Organomet. Chem., 2011, 696, 3155 CrossRef CAS.
- E. Alvarez, N. Guillou, C. Martineau, B. Bueken, B. Van de Voorde, C. Le Guillouzer, P. Fabry, F. Nouar, F. Taulelle, D. de Vos, J. Chang, K. Cho, N. Ramsahye, T. Devic, M. Daturi, G. Maurin and C. Serre, Angew. Chem., Int. Ed., 2015, 54, 3664 CrossRef CAS PubMed.
- B. Fillon, B. Lotz, A. Thierry and J. Wittmann, J. Polym. Sci., Part B: Polym. Phys., 1993, 31, 1395 CrossRef CAS.
- X. Li, K. Hu, M. Ji, Y. Huang and G. Zhou, J. Appl. Polym. Sci., 2002, 86, 633 CrossRef CAS.
- C. Landry, N. Pappe, M. Mason, A. Apblett, A. Tyler, A. MacInnes and A. R. Barron, J. Mater. Chem., 1995, 5, 331 RSC.
- M. Vougo-Zanda, J. Huang, E. Anokhina, X. Wang and A. Jacobson, J. Inorg. Chem., 2008, 47, 11535 CrossRef CAS PubMed.
- M. Murrie, S. Parsons and R. Winpenny, Chem. Commun., 1999, 285 RSC.
- R. C. Paul, O. B. Baidya, R. C. Kumar and R. Kapoor, Aust. J. Chem., 1976, 29, 1605 CrossRef CAS.
- J. P. Jolivet, Metal oxide Chemistry and Synthesis, John Wiley & Sons Ltd, 2000, p. 84 Search PubMed.
- X. H. Guan, G. H. Chen and C. Shang, J. Environ. Sci., 2007, 19, 438 CrossRef CAS.
- P. Persson, M. Karlsson and L. O. Öhman, Geochim. Cosmochim. Acta, 1998, 62(23/24), 3657 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Wide Angle X-ray Scattering (WAXS) data, FT-IR spectral data, Differential Scanning Calorimetric (DSC) analysis graphs, thermogravimetric analysis (TGA) plots and nitrogen sorption–desorption isotherms. See DOI: 10.1039/c5ra22764j |
| This journal is © The Royal Society of Chemistry 2016 |