Improved cycling performance of sulfur nanoparticles with a mussel inspired polydopamine coating†
Abstract
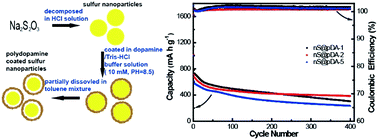
In this paper, a mussel inspired polydopamine (pDA) coating was successfully used to improve the electrochemical performance of sulfur nanoparticles. Sulfur nanoparticles with a pDA coating of a suitable thickness (i.e. 9.5 nm) could retain a capacity of 385.1 mA h g−1, 54% of the initial capacity at 0.5C (1C = 1670 mA g−1) after 400 charge/discharge cycles. Their cycling performance was greatly improved compared with that of uncoated sulfur nanoparticles due to the stabilization of sulfur nanoparticles by a pDA coating. Due to simplicity of the coating process and strong adhesion of the pDA coating, the process could be easily applied to the surface modification of other electrode materials.

 Please wait while we load your content...
Please wait while we load your content...