Activated carbon from Luffa cylindrica doped chitosan for mitigation of lead(ii) from an aqueous solution
Abstract
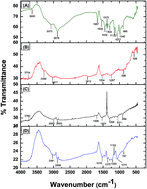
The present study is concerned with the batch adsorption of toxic lead(II) ions from an aqueous solution using activated carbon from a Luffa cylindrica fibers doped chitosan (ACLFCS) biocomposite as an adsorbent. The adsorption experiments were conducted as a function of pH, agitation time, initial lead(II) ion concentration and adsorbent dose. The synthesized biosorbent was characterized by instrumental techniques such as XRD, FTIR, SEM, BET surface area and BJH pore size distribution. XRD analysis revealed that the synthesized ACLFCS adsorbent exhibited broad diffraction peaks, reflecting an amorphous structure. FTIR study showed various functionalities, such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –OH and –NH2, which were responsible for lead(II) adsorption on the ACLFCS biocomposite. The surface morphology of the ACLFCS adsorbent possesses a porous texture with round- and elliptical-shaped voids that can provide adsorption sites for the adsorbate. BJH pore size distribution analysis showed an average pore diameter >2 nm for all chitosan (CS), activated carbon of Luffa cylindrica (ACLF) and ACLFCS adsorbents, corresponding to the presence of a mesoporous structure. Batch adsorption of lead(II) ions was carried out at room temperature wherein the optimum conditions for the maximum adsorption of lead(II) ions were attained at pH 5 with an adsorbent dose of 0.1 g L−1. The equilibrium adsorption isotherm data were fitted by the Langmuir and Freundlich models and the Langmuir isotherm exhibited the best fit with the experimental data. The maximum removal of lead(II) obtained was 98% (experimental) and 112 mg g−1 (from the Langmuir isotherm model). The adsorption kinetics was evaluated using pseudo-first-order, pseudo-second-order and intraparticle diffusion models. The adsorption data follows a pseudo-second-order kinetic model. The high uptake of lead(II) ions using ACLFCS indicates an effective and low cost adsorbent for the treatment of water contaminated with lead(II) ions.
O, –OH and –NH2, which were responsible for lead(II) adsorption on the ACLFCS biocomposite. The surface morphology of the ACLFCS adsorbent possesses a porous texture with round- and elliptical-shaped voids that can provide adsorption sites for the adsorbate. BJH pore size distribution analysis showed an average pore diameter >2 nm for all chitosan (CS), activated carbon of Luffa cylindrica (ACLF) and ACLFCS adsorbents, corresponding to the presence of a mesoporous structure. Batch adsorption of lead(II) ions was carried out at room temperature wherein the optimum conditions for the maximum adsorption of lead(II) ions were attained at pH 5 with an adsorbent dose of 0.1 g L−1. The equilibrium adsorption isotherm data were fitted by the Langmuir and Freundlich models and the Langmuir isotherm exhibited the best fit with the experimental data. The maximum removal of lead(II) obtained was 98% (experimental) and 112 mg g−1 (from the Langmuir isotherm model). The adsorption kinetics was evaluated using pseudo-first-order, pseudo-second-order and intraparticle diffusion models. The adsorption data follows a pseudo-second-order kinetic model. The high uptake of lead(II) ions using ACLFCS indicates an effective and low cost adsorbent for the treatment of water contaminated with lead(II) ions.


 Please wait while we load your content...
Please wait while we load your content...