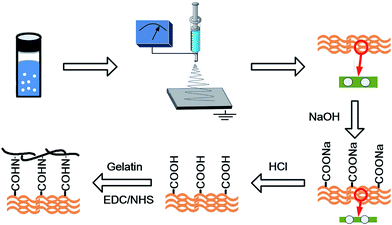
Fabrication and characterization of PCL/CaCO3 electrospun composite membrane for bone repair†
Zhinan Caoab,
Dandan Wangab,
Lingwei Lyuab,
Yihong Gong*ab and
Yan Li*ab
aDepartment of Biomedical Engineering, School of Engineering, Sun Yat-sen University, Guangzhou, Guangdong, P.R. China. E-mail: gongyih@mail.sysu.edu.cn; liyan99@mail.sysu.edu.cn; Fax: +86-20-39332146; Fax: +86-20-39387890; Tel: +86-20-39332146 Tel: +86-20-39387890
bGuangdong Provincial Key Laboratory of Sensor Technology and Biomedical Instrument, Sun Yat-sen University, Guangzhou, Guangdong, P.R. China
First published on 14th January 2016
Abstract
Tissue engineering offers a promising approach to repair bone defects, of which a scaffold is an indispensable component. An ideal scaffold should mimic the organic and inorganic compositions of bone. Here, CaCO3/casein microspheres were encapsulated in PCL composite membranes using co-solvent electrospinning to mimic the hierarchical structure and composition of bone ECM. As PCL lacks functional groups to support cell adhesion, gelatin was grafted onto membranes. To find the optimum composition, CaCO3/casein microspheres were entrapped at five different concentrations. Various analytical techniques, including FTIR, XRD, SEM and EDS, were applied to characterize the particles and membranes. The CaCO3/casein microparticles were spheres of ~1 μm, mainly in vaterite form. The amount of casein was 23.9 ± 1% determined by BCA assay and its presence stabilized CaCO3 in vaterite. After surface modification, the hydrophilicity of membranes was improved while the membrane morphology was not significantly changed; on the membranes, both gelatin and CaCO3/casein microspheres were evenly distributed. Due to the presence of vaterite, the biomineralization property of a composite membrane was significantly enhanced. Furthermore, we compared HMSC proliferation on composite membranes with FDA staining and MTT assay. After cells were cultured in osteogenic medium, differentiation potential was investigated by analyzing gene expressions of RUNX2, COL-I and ALP, and monitoring ALP activity. Presence of CaCO3/casein microspheres enhanced cell proliferation and differentiation, especially sample P-20 which demonstrated better potential to be used in bone tissue engineering than others.
1. Introduction
Bone is a dynamic tissue with unique mechanical and biological properties.23 It mainly consists of organic collagen type I fibers and inorganic hydroxyapatite (HAp) crystals in a well organized nanostructure.16 It may be impaired and inactivated by diseases or accidents. Thus, bone grafts are widely needed to replace insufficient amounts of bone in order to repair bone defects.11,28,41 Millions of bone transplantations are performed annually in the world,2,18 making a great clinical need for bone grafts.2 Limited by a shortage of tissue donors, tissue engineering is an alternative and promising approach which can be developed with combinations of scaffolds, cells and bioactive agents to reproduce and repair damaged tissue.20,30,45,47In order to develop an effective strategy for bone regeneration, the scaffolds should mimic the hierarchical structure and biological functions of natural extracellular matrices (ECM) of bone, which is of organic/inorganic composition. Among various scaffolds,3,4,37,42,44 electrospinning fibrous membranes offer great advantages such as mimicking natural ECM; in addition, they can be fabricated with a simple method and are porous.31 There is a wide range of polymers such as poly(lactic acid) (PLA),29,30,46 poly(lactic-co-glycolic acid) (PLGA)33 and poly(ε-caprolactone) (PCL)32 which can be fabricated as fibrous membranes. More specifically, PCL has advantages of biocompatibility, processability and degradability.6,32 However, it lacks bioactivity such as osteoconductivity.
Recent research efforts have been focused on incorporation of inorganic particles within the polymeric matrix to prepare superior materials as bone substitutes.6 There are various inorganic fillers, such as HAp,16 bioactive glass,1 CaCO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 and TiO2.14 Among them, CaCO3, with advantages of biocompatibility and a simple synthesizing method, has been used in multi-purpose applications.36,38,39 CaCO3 has three anhydrous crystalline forms: calcite, aragonite and vaterite. Calcite is the most thermodynamically stable form. However, for applications such as bone cavity filling biomaterials, the ideal form is vaterite because of its special structure and unique character such as high dispersion and high specific surface area. The vaterite form is not thermodynamically stable and easy to transform into rhombohedral calcite,21 thus organic additives are needed to stabilize the vaterite form. Various polymers have been applied to affect crystallization of CaCO3, such as acrylic acid polymers19 and poly(sodium 4-styrenesulfonate).40 However, none of these additives have potential to demonstrate osteoconductivity or to improve the bioactivity of CaCO3.
34 and TiO2.14 Among them, CaCO3, with advantages of biocompatibility and a simple synthesizing method, has been used in multi-purpose applications.36,38,39 CaCO3 has three anhydrous crystalline forms: calcite, aragonite and vaterite. Calcite is the most thermodynamically stable form. However, for applications such as bone cavity filling biomaterials, the ideal form is vaterite because of its special structure and unique character such as high dispersion and high specific surface area. The vaterite form is not thermodynamically stable and easy to transform into rhombohedral calcite,21 thus organic additives are needed to stabilize the vaterite form. Various polymers have been applied to affect crystallization of CaCO3, such as acrylic acid polymers19 and poly(sodium 4-styrenesulfonate).40 However, none of these additives have potential to demonstrate osteoconductivity or to improve the bioactivity of CaCO3.
Casein, the most abundant milk protein, demonstrated the potential to enhance biomineralization. It is a proline-rich phosphoprotein, possessing four forms: αS1-, αS2-, β- and κ-casein.17 They are all in amphiphilic structures,8 which preserve the ability to interact with a wide range of active compounds via functional groups on their primary polypeptide structure, offering a variety of possibilities for reversible binding with active molecules such as Ca and also enhancing Ca absorption.9,43 β-casein was used here to stabilize the crystallization of CaCO3 in order to fabricate vaterite.
In this study, to mimic the hierarchical structure and biological functions of ECM, CaCO3/casein microspheres were encapsulated in PCL using a co-solvent-based electrospinning method26 to fabricate composite fibrous membranes with osteoconductivity (Scheme 1). PCL is a synthetic biomaterial and lacks functional groups which makes membranes difficult to support cell adhesion.25 Thus, we used gelatin for surface modification.10,22 To find the optimum composition of a membrane, CaCO3/casein microspheres were entrapped at five different concentrations. Several analytical techniques, including Fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD), scanning electron microscopy (SEM), and energy dispersive spectroscopy (EDS), were applied to characterize the particles and membranes. Furthermore, we compared cell proliferation and differentiation potential on various composite membranes with fluorescein diacetate (FDA) staining, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay, gene expression analysis of transcription factors Runt-related transcription factor 2 (RUNX2), collagen type I (COL-I) and alkaline phosphatase (ALP), and also ALP activity test.
2. Results and discussion
2.1 Characterization of microparticles and composite membranes
In this study, CaCO3/casein hybrid microparticles were fabricated using a co-precipitation method.43 Several analytical techniques were employed to characterize the microparticles. As shown in Fig. 1a, there are seven main XRD diffraction peaks assigned to (004), (110), (112), (114), (300), (118) and (224) planes of vaterite (JCPDS card No. 74-1867). Additionally, three small diffraction peaks of calcite (JCPDS Card No. 86-0174) were observed. All vaterite peaks are very broad, indicating CaCO3/casein microparticles were aggregates of nanoparticles. Functional groups in samples were investigated using FTIR (Fig. 1b). The characteristic vaterite band at 751 cm−1 was present12 and the CO32− bands were found at 870 cm−1 and 1640 cm−1.24,35 In addition, due to the presence of casein, –CONH– absorption band at 1650 cm−1, –NH– band at 1541 cm−1, and C–H band at 3400 cm−1 were all present in the spectrum.43 However, even though calcite diffraction peaks were shown in the XRD pattern, there was no absorption band at 1800 cm−1, which belongs to calcite crystals.13 The morphology of CaCO3/casein microparticles was characterized by SEM (Fig. 1c). The size was measured using Image J software and its distribution was shown in Fig. 1d. All microparticles were in a spherical shape of ~1 μm, and its size distribution was very narrow. Even though calcite diffraction peaks were present in the XRD pattern, calcite particles, which were usually rhombohedral, were not observed.The amount of casein in the as-prepared particles was quantified using a BCA protein assay kit, where it was determined to be 23.9 ± 1% with an encapsulation efficiency of 38.76 ± 1.6%. We speculated that the presence of casein influenced particle formation and also stabilized particles in vaterite form. Organic additives have been proved to be absorbed onto ionic crystals and preceded over all other crystal nucleus surfaces, and thus effectively precluded the crystal growth.9 Here, casein was supposed to have two functions: on one hand, binding to the growth sites of the crystals in order to inhibit crystal growth, and on the other hand acting as a heterogeneous nucleator to control and stabilize the precipitating polymorph.5
CaCO3/casein microparticles were entrapped inside PCL fibers through electrospinning to form a composite membrane. Since PCL was hydrophobic and lacked functional groups for cell adhesion, the membranes were first treated with NaOH solution to partially hydrolyze PCL and then gelatin was grafted. When looking at P-50 as an example, the water contact angle of as-electrospun membrane was 113.9 ± 3.7°, while after NaOH treatment, the value decreased to 76.3 ± 1.3°. Even though the presence of NaOH partially degraded PCL, based on SEM observation, the membrane morphology was not significantly changed. The fibers were of similar diameters as that before NaOH treatment, although the entrapped CaCO3/casein microparticles were exposed (ESI, Fig. S1†). After the surface modification process as described in Section 4.2.3, the water contact angle was 66.4 ± 6.0°.
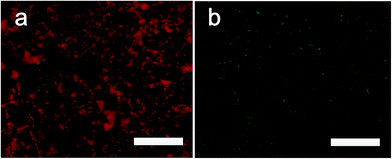
To observe the distribution of gelatin and casein on membranes, we used Rhodamine B labeled gelatin and fluorescein isothiocyanate (FITC) labeled casein for surface modification. Casein has strong affinity with Ca. Thus, relying on the casein, we can also tell the distribution of CaCO3/casein microparticles in membrane. As shown in Fig. 2, both gelatin and these microparticles were evenly distributed while lots of CaCO3/casein were present on the surface since some green fluorescence dots were observed.
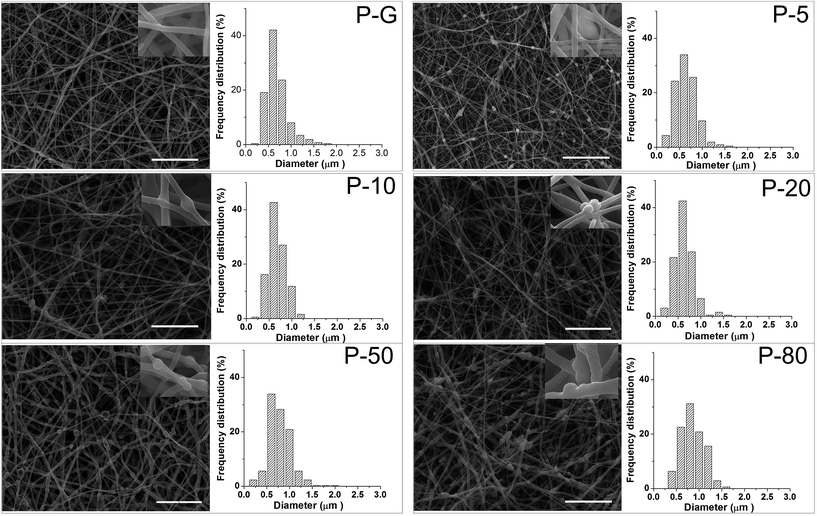
The morphology of composite membranes was examined using SEM. As shown in Fig. 3, there were no beads in the fiber (insets of each sample) and CaCO3/casein microspheres were evenly distributed across the membranes. The fiber diameters of different samples were quantified with Image J software, where the diameters primarily ranged from 400 nm to 1000 nm. However, for sample P-80, due to the very high amount of CaCO3/casein, fiber diameters were slightly larger, ranging from 400 nm to 1200 nm. The average fiber diameters were 476 ± 176 nm, 518 ± 250 nm, 537 ± 211 nm, 646 ± 151 nm, 687 ± 261 nm and 753 ± 245 nm for P-G, P-5, P-10, P-20, P-50 and P-80, respectively. With an increase of CaCO3/casein amounts, the fiber diameters showed an increasing trend but the difference was not significant.
In vitro biomineralization properties of composite membranes after NaOH treatment were first evaluated by soaking in simulated body fluid (SBF) at 37 °C for 21 days during which the SBF solution was not exchanged. Then samples were taken out for SEM, XRD and FITR analysis. As shown in Fig. S2,† there was significantly more crystal deposition on the membrane with CaCO3/casein than PCL membrane and PCL membrane with calcite. After mineralization, two additional diffraction peaks were observed in the XRD pattern, and these two peaks were assigned to HAp (Fig. S3b†); the absorption band of PO4 was observed in FTIR spectrum (Fig. S3a†) and EDS spectrum also showed a large amount of the element phosphorus (Fig. S3c†). Based on these results, we can conclude that the presence of CaCO3/casein vaterite particles enhanced the biomineralization of composite membranes.
For better mimicking ECM, modifications of membrane surfaces were conducted. PCL is inert6 (cells were round shaped on membranes without surface modification as shown in Fig. S4†), so we modified the surface with cross-linking gelatin.10,22 Representative samples P-G, P-20, P-50, and P-80 were selected for in vitro biomineralization evaluation. After immersion in SBF for 7 days, various amounts of crystals were deposited on different samples (Fig. 4). For P-G and P-20 membranes, only a few crystals were found in the pores of membranes in SEM images at larger magnification (Fig. 4, 2nd column). With an increase of CaCO3/casein microspheres, a thin layer of crystals was observed on P-50 and P-80 surfaces. On the P-50 surface, crystal agglomerates were in a spherical size of ∼10 μm, while on the P-80 surface, the agglomerates were much larger and their amount was much higher. Hence the enhancing effect on biomineralization was improved after surface modifications and the biomineralization potential of composite membranes increased with CaCO3/casein vaterite amounts.
 | ||
| Fig. 4 Representative SEM images of composite fibrous membranes after soaking in SBF for 7 days. The left scale bar is 40 μm and the right scale bar is 2 μm. | ||
2.2 Cell adhesion and proliferation test
Cell attachment and spreading on scaffolds is a crucial requirement for the following cell activities. After FDA staining (Fig. 5), on all membranes cells were spreading very well and in a spindle shape. It seemed that more cells were on P-20 and P-50 membranes than others. Furthermore, cell proliferation was investigated with MTT assay. As shown in Fig. 6, the UV absorbance increased for all composite membranes from day 1 to 7, indicating the surface modification strategy was beneficial for cell proliferation. Among all samples, P-20 and P-50 membranes demonstrated higher absorbance values than the other membranes from day 3 to day 7, especially on day 7.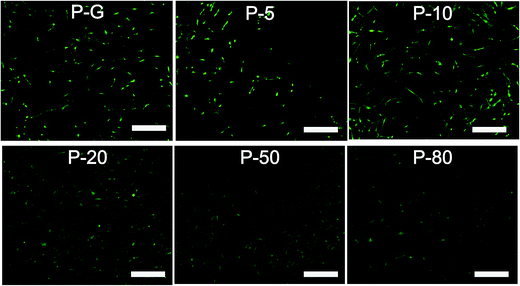 | ||
| Fig. 5 Fluorescence microscope images of human mesenchymal stem cells (HMSCs) on composite membranes after FDA staining on day 5. The scale bar is 200 μm. | ||
2.3 Cell differentiation investigation
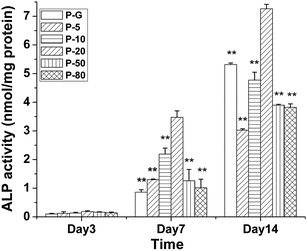
CaCO3 and casein have been proven to enhance calcification and differentiation of cells. The effects of composite membranes on differentiation of HMSCs were investigated here. The relative expression of osteogenic marker genes was analyzed by RT-PCR. As shown in Fig. 7, the expression of ALP, COL-I and RUNX-2 genes was the highest in HMSCs on P-20 membrane. Furthermore, the change of ALP activity of HMSCs on different membranes with differentiation time is shown in Fig. 8. From day 3 to 14, the ALP activity increased for all membranes, with the group P-20 demonstrating peak values on days 7 and 14. When looking at ALP expression, it was interesting to find that the sample trend from the highest to the lowest relative levels of gene expressions was nearly the same as that based on its activity on the same day. This consistence confirmed that our differentiation evaluation was conducted well. Thus, we can conclude that P-20 membrane showed the highest effect to enhance HMSCs osteogenic differentiation.Both proliferation and differentiation studies demonstrated that the enhancing effect did not increase with concentration of CaCO3/casein particles in composite membranes. This was probably because high amounts of CaCO3/casein would produce a rich Ca2+ microenvironment which exhibits negative effects on cells. Nevertheless, our results thus far showed that the composite membrane P-20 showed a potential towards developing an optimal osteoinductive and osteoconductive system.
3. Conclusions
In this study, CaCO3/casein hybrid microparticles were fabricated and further entrapped in PCL composite membranes to improve in vitro biomineralization, cell proliferation and differentiation properties. To find the optimum composition of membranes, CaCO3/casein microspheres were entrapped at five different concentrations. The CaCO3/casein microparticles were mainly in the vaterite form based on XRD investigation. The presence of casein in microparticles was confirmed by FTIR analysis and BCA assay, where its content was determined to be 23.9 ± 1%. Casein affected the crystallization and stabilization of CaCO3 in vaterite. All vaterite microparticles were spherical of ~1 μm, and their size distribution was very narrow. Since PCL was hydrophobic and lacked functional groups for cell adhesion, surface modification of composite membranes was conducted. For sample P-50, the water contact angle decreased from 113.9 ± 3.7° to 66.4 ± 6.0°, the membrane morphology was not significantly changed with similar fiber diameter as that before surface modification, and both gelatin and casein were evenly distributed on the membrane. Due to the presence of vaterite microparticles, the biomineralization property of the composite membrane was significantly enhanced, where the amount of HAp deposition increased with vaterite content. On composite membranes, HMSCs were spread as spindles and proliferated very well, especially on sample P-20 and P-50. As for gene expressions of ALP, COL-I and RUNX-2 after cells induced to differentiation, the presence of CaCO3/casein particles improved gene expressions, especially sample P-20 which had the highest level and also the highest ALP activity. Based on our studies, P-20 demonstrated better potential to be applied in bone tissue engineering and the in vivo performance will be conducted in the future.4. Experimental
4.1 Materials
Casein, dexamethasone, 3-(3-dimethylaminopropyl)-1-ethyl-carbodiimide hydrochloride (EDC), FDA, N-hydroxy-succinimide (NHS), Rhodamine B, FITC, β-glycerophosphate, L-ascorbic acid, and PCL (Mw = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–90
000–90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) were purchased from Sigma-Aldrich (St, Louis, MO, USA). Sodium carbonate (Na2CO3), calcium chloride (CaCl2), dichloromethane (DCM, 99.5%), dimethyl formamide (DMF, 99.5%), dimethyl sulfoxide (DMSO, 99.0%), and ethanol were obtained from Guangzhou Chemical Reagent Co. (Guangzhou, Guangdong, China) and used without further purification. SBF was prepared following procedures as reported elsewhere7 and all chemicals were from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China). Alpha minimum essential medium (α-MEM) and PS (penicillin/streptomycin antibiotics) were purchased from HyClone (Logan, UT, USA). Fetal bovine serum (FBS) and trypsin–EDTA were purchased from GIBICO (Carlsbad, CA, USA). MTT and gelatin were from Amresco (Solon, OH, USA) and the ALP assay kit was from Beyotime (Haimen, Jiangsu, China). The Trizol reagent kit was from Invitrogen (CA, USA), the PrimeScript RT reagent kit was from TaKaRa (Mountain View, CA, USA), and GoTaq qPCR Master Mix was from Promega (Madison, WI, USA). Deionized (DI) ultrapure water was used throughout the experiment.
000) were purchased from Sigma-Aldrich (St, Louis, MO, USA). Sodium carbonate (Na2CO3), calcium chloride (CaCl2), dichloromethane (DCM, 99.5%), dimethyl formamide (DMF, 99.5%), dimethyl sulfoxide (DMSO, 99.0%), and ethanol were obtained from Guangzhou Chemical Reagent Co. (Guangzhou, Guangdong, China) and used without further purification. SBF was prepared following procedures as reported elsewhere7 and all chemicals were from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China). Alpha minimum essential medium (α-MEM) and PS (penicillin/streptomycin antibiotics) were purchased from HyClone (Logan, UT, USA). Fetal bovine serum (FBS) and trypsin–EDTA were purchased from GIBICO (Carlsbad, CA, USA). MTT and gelatin were from Amresco (Solon, OH, USA) and the ALP assay kit was from Beyotime (Haimen, Jiangsu, China). The Trizol reagent kit was from Invitrogen (CA, USA), the PrimeScript RT reagent kit was from TaKaRa (Mountain View, CA, USA), and GoTaq qPCR Master Mix was from Promega (Madison, WI, USA). Deionized (DI) ultrapure water was used throughout the experiment.
4.2 Preparation and characterization of the fibrous membranes
The crystal phase and functional groups of the as-synthesized product were analyzed with XRD and FTIR spectroscopy. Surface morphology and particle size were investigated using a SEM (JEOL JSM-5600) with an accelerating voltage and a beam current at 15 kV and 10 mA, respectively. The casein content was measured with a BCA protein assay kit (Thermo Scientific Pierce, Rickford II, USA).
To demonstrate the distribution of last adsorbed casein and gelatin on the membranes, the incubation process was performed using FITC labeled casein15 and Rhodamine B labeled gelatin.27 During the process, an Olympus IX71 microscope (Olympus, Tokyo, Japan) was used to capture the fluorescent images and processed by Image-ProPlus software (Media Cybernetics, Rockville, MD, USA).
4.3 In vitro biomineralization assessment
Biomineralization properties of the membranes were evaluated by soaking in SBF at 37 °C. The SBF was changed every 2 days, and after 7 days samples were removed from the medium, gently rinsed with DI water and dried at room temperature. The SEM images were taken to observe the surface morphology.4.4 Cell adhesion assay
Before cell seeding, membranes were placed in 24-well tissue culture plates and sterilized by soaking in 75% alcohol and exposed to UV radiation for 30 min, then soaked in 10× PS for 12 h and washed 3 times with PBS. HMSC were seeded onto the composite membranes at a density of 8000 mL−1 and cultured with basal growth medium which was α-MEM containing 10% FBS at 37 °C in 5% CO2 humidified atmosphere for 7 days. The culture medium was changed every 2 days. On day 5, cells were washed with PBS and then incubated in FDA (5 μg mL−1) solution at 37 °C for 10 min. After rinsing with PBS, fluorescent images were captured by an Olympus IX71 microscope (Olympus, Tokyo, Japan).During the 7 days of culturing, MTT assay was used to investigate cellular proliferation. Specifically, on days 1, 3, 5 and 7, 300 μL MTT (0.5 mg mL−1) solution was added to each well and incubated at 37 °C for 4 h in the dark; then 500 μL of DMSO was added to each well to dissolve the purple formazan crystals. The solution was transferred to a 96-well plate and the absorbance was measured at 570 nm using a microplate reader (BioTek Synergy4, USA).
4.5 In vitro osteoconductivity evaluation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per mL) than that for proliferation studies. After culturing for 2 days in basal growth medium, HMSCs were induced to differentiation in osteogenic medium which was basal growth medium supplemented with 10 mM β-glycerophosphate, 0.2 mM L-ascorbic acid and 0.1 μM dexamethasone for 14 days.
000 cells per mL) than that for proliferation studies. After culturing for 2 days in basal growth medium, HMSCs were induced to differentiation in osteogenic medium which was basal growth medium supplemented with 10 mM β-glycerophosphate, 0.2 mM L-ascorbic acid and 0.1 μM dexamethasone for 14 days.The quantification of gene expressions was carried out by RT-PCR using 20 ng of cDNA and GoTaq qPCR Master Mix following manufacturer's procedures. The primers were previously designed using the Primer 3 online software and synthesized by Invitrogen. The primer sequences of osteogenesis-related genes including COL-I, ALP, RUNX2 and the endogenous control gene GAPDH are listed in Table 1. Expression of these genes was analyzed from the cDNA (20 ng) and performed with the ABI 7500 Real-time PCR Detector (Applied Biosystems, Foster City, CA, USA) and quantified via the X = 2−ΔΔCt method, in which ΔΔCt = ΔE − ΔC, ΔE = Ct exp − Ct GAPDH and ΔC = Ct ctr1 − Ct GAPDH.
| Gene | Primer sequence (5′–3′) | Gene bank access number |
|---|---|---|
| GAPDH | F: AGAAAAACCTGCCAAATATGATGAC | NM_002046 |
| R: TGGGTGTCGCTGTTGAAGTC | ||
| COL-I | F: CAGCCGCTTCACCTACAGC | NM_000088.3 |
| R: TTTTGTATTCAATCACTGTCTTGCC | ||
| ALP | F: AGCACTCCCACTTCATCTGGAA | NM_000478.3 |
| R: GAGACCCAATAGGTAGTCCACATTG | ||
| RUNX2 | F: AGAAGGCACAGACAGAAGCTTGA | NM_001015051.3 |
| R: AGGAATGCGCCCTAAATCACT |
4.6 Statistical analysis
Data are presented as mean ± standard deviation (SD) and were analyzed by one-way analysis of variance (ANOVA) test. p < 0.05 was considered to indicate a statistically significant difference.Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (51303216, 51103182 and 51203194), Guangdong Provincial Education Department (2013KJCX005), Science and Technology Planning Project of Guangdong Province (2011A060901013), and Guangdong Innovative Research Team Program (2009010057).Notes and references
- B. A. Allo, S. G. Lin, K. Mequanint and A. S. Rizkalla, ACS Appl. Mater. Interfaces, 2013, 5, 7574–7583 CAS.
- D. Bhuiyan, M. J. Jablonsky, I. Kolesov, J. Middleton, T. M. Wick and R. Tannenbaum, Acta Biomater., 2015, 15, 181–190 CrossRef CAS PubMed.
- S. G. Caridade, C. Monge, J. Almodovar, R. Guillot, J. Lavaud, V. Josserand, J.-L. Coll, J. F. Mano and C. Picart, Acta Biomater., 2015, 15, 139–149 CrossRef CAS PubMed.
- J. S. Carson and M. P. G. Bostrom, Injury, 2007, 38, S33–S37 CrossRef PubMed.
- K. D. Cashman, Br. J. Nutr., 2002, 87, S169–S177 CrossRef CAS PubMed.
- F. Chen, C. N. Lee and S. H. Teoh, Mater. Sci. Eng., C, 2007, 27, 325–332 CrossRef CAS.
- A. Cuneyt Tas, J. Non-Cryst. Solids, 2014, 400, 27–32 CrossRef CAS.
- C. G. de Kruif, T. Huppertz, V. S. Urban and A. V. Petukhov, Adv. Colloid Interface Sci., 2012, 171–172, 36–52 CrossRef CAS PubMed.
- A. O. Elzoghby, W. S. El-Fotoh and N. A. Elgindy, J. Controlled Release, 2011, 153, 206–216 CrossRef CAS PubMed.
- M. Foox, A. Raz-Pasteur, I. Berdicevsky, N. Krivoy and M. Zilberman, Polym. Adv. Technol., 2014, 25, 516–524 CrossRef CAS.
- M. S. Fourman, E. W. Borst, E. Bogner, S. R. Rozbruch and A. T. Fragomen, Clin. Orthop. Relat. Res., 2014, 472, 732–739 CrossRef PubMed.
- M. Fujiwara, K. Shiokawa, K. Morigaki, Y. C. Zhu and Y. Nakahara, Chem. Eng. J., 2008, 137, 14–22 CrossRef CAS.
- Y. P. Guo, Y. Zhou, D. C. Jia and H. X. Tang, Microporous Mesoporous Mater., 2009, 118, 480–488 CrossRef CAS.
- K. K. Gupta, A. Kundan, P. K. Mishra, P. Srivastava, S. Mohanty, N. K. Singh, A. Mishra and P. Maiti, Phys. Chem. Chem. Phys., 2012, 14, 12844–12853 RSC.
- J. Habibi, S. L. Brandt, T. A. Coudron, R. M. Wagner, M. K. Wright, E. A. Backus and J. E. Huesing, Arch. Insect Biochem. Physiol., 2002, 50, 62–74 CrossRef CAS PubMed.
- M. I. Hassan, N. Sultana and S. Hamdan, J. Nanomater., 2014, 573238 Search PubMed.
- D. S. Horne, Curr. Opin. Colloid Interface Sci., 2006, 11, 148–153 CrossRef CAS.
- D. W. Hutmacher, Biomaterials, 2000, 21, 2529–2543 CrossRef CAS PubMed.
- S. Kim and C. B. Park, Adv. Funct. Mater., 2013, 23, 10–25 CrossRef CAS.
- Y. Koo, H. Lee, S. Kim, N. J. Song, J. M. Ku, J. Lee, C. H. Choi, K. W. Park and G. Kim, RSC Adv., 2015, 5, 44943–44952 RSC.
- A. López-Marzo, J. Pons and A. Merkoçi, J. Mater. Chem., 2012, 22, 15326 RSC.
- H. Layman, M. G. Spiga, T. Brooks, S. Pham, K. A. Webster and F. M. Andreopoulos, Biomaterials, 2007, 28, 2646–2654 CrossRef CAS PubMed.
- S. S. Lee, B. J. Huang, S. R. Kaltz, S. Sur, C. J. Newcomb, S. R. Stock, R. N. Shah and S. I. Stupp, Biomaterials, 2013, 34, 452–459 CrossRef CAS PubMed.
- P. Liang, D. Zhao, C.-Q. Wang, J.-Y. Zong, R.-X. Zhuo and S.-X. Cheng, Colloids Surf., B, 2013, 102, 783–788 CrossRef CAS PubMed.
- C.-H. Lin, S.-h. Hsu, J.-M. Su and C.-W. Chen, J. Tissue Eng. Regener. Med., 2011, 5, 156–162 CrossRef CAS PubMed.
- A. Martins, A. R. C. Duarte, S. Faria, A. P. Marques, R. L. Reis and N. M. Neves, Biomaterials, 2010, 31, 5875–5885 CrossRef CAS PubMed.
- K. Morimoto, S. Chono, T. Kosai, T. Seki and Y. Tabata, J. Pharm. Pharmacol., 2005, 57, 839–844 CrossRef CAS PubMed.
- P. M. Mountziaris, S. N. Tzouanas and A. G. Mikos, Biomaterials, 2010, 31, 1666–1675 CrossRef CAS PubMed.
- G. Pitarresi, C. Fiorica, F. S. Palumbo, F. Calascibetta and G. Giammona, J. Biomed. Mater. Res., Part A, 2012, 100A, 1565–1572 CrossRef CAS PubMed.
- J. Pu, F. Yuan, S. Li and K. Komvopoulos, Acta Biomater., 2015, 13, 131–141 CrossRef CAS PubMed.
- I. Rajzer, E. Menaszek, R. Kwiatkowski, J. A. Planell and O. Castano, Mater. Sci. Eng., C, 2014, 44, 183–190 CrossRef CAS PubMed.
- M. G. Raucci, V. D'Anto, V. Guarino, E. Sardella, S. Zeppetelli, P. Favia and L. Ambrosio, Acta Biomater., 2010, 6, 4090–4099 CrossRef CAS PubMed.
- C. Ru, F. Wang, M. Pang, L. Sun, R. Chen and Y. Sun, ACS Appl. Mater. Interfaces, 2015, 7, 10872–10877 CAS.
- S. Stocks-Fischer, J. K. Galinat and S. S. Bang, Soil Biol. Biochem., 1999, 31, 1563–1571 CrossRef CAS.
- W. L. Suchanek, P. Shuk, K. Byrappa, R. E. Riman, K. S. TenHuisen and V. F. Janas, Biomaterials, 2002, 23, 699–710 CrossRef CAS PubMed.
- S. Tugulu, R. Barbey, M. Harms, M. Fricke, D. Volkmer, A. Rossi and H. A. Klok, Macromolecules, 2007, 40, 168–177 CrossRef CAS.
- S. G. Vallejo-Heligon, B. Klitzman and W. M. Reichert, Acta Biomater., 2014, 10, 4629–4638 CrossRef CAS PubMed.
- D. V. Volodkin, S. Schmidt, P. Fernandes, N. I. Larionova, G. B. Sukhorukov, C. Duschl, H. Mohwald and R. von Klitzing, Adv. Funct. Mater., 2012, 22, 1914–1922 CrossRef CAS.
- C. Y. Wan and B. Q. Chen, Biomed. Mater., 2011, 6, 055010 CrossRef PubMed.
- Y. Wang, Y. X. Moo, C. Chen, P. Gunawan and R. Xu, J. Colloid Interface Sci., 2010, 352, 393–400 CrossRef CAS PubMed.
- A. M. Wojtowicz, A. Shekaran, M. E. Oest, K. M. Dupont, K. L. Templeman, D. W. Hutmacher, R. E. Guldberg and A. J. Garcia, Biomaterials, 2010, 31, 2574–2582 CrossRef CAS PubMed.
- G. Xu, X. Wang, C. Deng, X. Teng, E. J. Suuronen, Z. Shen and Z. Zhong, Acta Biomater., 2015, 15, 55–64 CrossRef CAS PubMed.
- Z. Xu, G. Liang, L. Jin, Z. Wang, C. Xing, Q. Jiang and Z. Zhang, J. Cryst. Growth, 2014, 395, 116–122 CrossRef CAS.
- M. Yamamoto, Y. Takahashi and Y. Tabata, Tissue Eng., 2006, 12, 1305–1311 CrossRef CAS PubMed.
- A. M. Yousefi, M. E. Hoque, R. Prasad and N. Uth, J. Biomed. Mater. Res., Part A, 2015, 103, 2460–2481 CrossRef CAS PubMed.
- A. Zajicova, E. Javorkova, P. Trosan, M. Krulova and V. Holan, J. Tissue Eng. Regener. Med., 2014, 8, 277–278 Search PubMed.
- X. Zhang, V. Thomas and Y. K. Vohra, J. Mater. Sci.: Mater. Med., 2010, 21, 541–549 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: In SEM images, it was shown that NaOH treatment resulted in exposure of the entrapped CaCO3/casein microparticles, while there was no significant change in fiber diameter; comparing CaCO3/casein PCL composite membrane with PCL membrane and calcite PCL composite membrane, SEM images showed that there was significantly more crystal deposition on PCL membrane with CaCO3/casein, while XRD, FITR and EDS analysis confirmed that the deposited crystal was HAp; the cells morphology changed from round to spindle after surface modification. See DOI: 10.1039/c5ra22548e |
| This journal is © The Royal Society of Chemistry 2016 |