Cryptotanshinone inhibits proliferation and induces apoptosis via mitochondria-derived reactive oxygen species involving FOXO1 in estrogen receptor-negative breast cancer Bcap37 cells
Xiaoman Liuab,
Lili Pana,
Junling Lianga,
Jinhui Lic and
Shihua Wu*a
aResearch Center of Siyuan Natural Pharmacy and Biotoxicology, College of Life Sciences, Zhejiang University, Hangzhou 310058, China. E-mail: drwushihua@zju.edu.cn; Fax: +86-571-88206287; Tel: +86-571-88206287
bSchool of Medicine, Shandong University, Jinan 250021, China
cInstitute of Agrobiology and Environmental Sciences, Zhejiang University, Hangzhou 310058, China
First published on 17th February 2016
Abstract
Cryptotanshinone is a natural abietane type-diterpene quinine isolated from the lipophilic extract of Tanshen (or Danshen, Salvia miltiorrhiza Bunge) which is widely used in clinical applications for treating inflammations such as acne, tonsillitis and mastitis at present. Previous studies indicated that cryptotanshinone could not only inhibit the growth of androgen dependent and castration resistant prostate cancer cells by suppressing the androgen receptor (AR), but also inhibit estrogen receptor (ER)-positive breast cancer cell proliferation by suppressing ER signals. In this work, we found cryptotanshinone can effectively inhibit the proliferation of ER-negative breast cancer Bcap37 cells. Cryptotanshinone induced mitochondria-mediated apoptosis through changes in nuclear morphology, DNA fragmentation, loss of mitochondrial membrane potential, activation of caspase-like activities and translocation of endonuclease G (EndoG) from mitochondria into nucleus. During the process, the caspases inhibitor could not completely abrogate apoptosis caused by cryptotanshinone, suggesting that the intrinsic caspase-independent signaling functioned was one of the major pathways under high stress of cryptotanshinone. In addition, the apoptosis involved several key signals of cell proliferation and ROS regulation, such as suppression of PTEN and up-regulation of phosphorylated-AKT (Ser 473), thus the expression of a key transcription factor FOXO1 was down-regulated further and resulted in accumulation of ROS that brought about the following oxidative DNA damage. In summary, the results showed that cryptotanshinone might be a promising apoptosis-inducing agent in the treatment of ER-negative breast cancers by activation of mitochondria-derived ROS/FOXO1 signaling pathways.
1. Introduction
Although there are significant advancements in human health prevention and treatment, breast cancer is still the most frequently diagnosed cancer and is also the second worldwide leading cause of cancer death in females particularly in western countries.1,2 During the past few years, receptor-targeting therapeutic strategies have been proved to be efficient to prolong survival for breast cancer patients with estrogen receptor (ER), progesterone receptor (PR), and or human epidermal growth factor receptor 2 (HER-2). ER was found to be a major receptor of breast cancer, and about 75% of breast cancers are ER- or PR-positive.3–6 Thus, deprivation of estrogenic signaling pathways has been the main and key therapeutic form of hormonal therapy for patients with ER-positive and/or PR-positive diseases both pre- and postmenopausal patients.7–9 However, definitive treatments of receptor-negative breast cancer including surgical resection, radiation, conventional chemotherapy, and other systemic cures still face significant challenges owing to recurrence, acquired resistance and genomic instability of cancer cells. Therefore, breast cancer especially for ER-negative cancer and triple-negative breast cancer still takes a tremendous toll, and screening new and more effective prevention and chemotherapeutics are urgently needed.Natural products have been used as one of the most important resources for new drug developments, especially for anti-cancer and anti-inflammatory drugs.10–12 In the course of screening new anti-cancer natural products, we found that tanshinones have potent cytotoxic activities for several cancer cells in vitro and several high pure tanshinones from a famous Traditional Chinese Medicine Tanshen (or Danshen, Salvia miltiorrhiza Bunge) were prepared by use of unique liquid–liquid counter-current chromatography methods.13–16 It has been known that tanshinones were the most prominent lipophilic diterpenoids17 and possess potent pharmacological activities such as antibacterial, antioxidant, anti-inflammatory and antineoplastic.18,19 Up to now, about 40 tanshinones have been isolated from the plant S. miltiorrhiza Bunge.17,19
Among the tanshinones, cryptotanshinone as one of the major constituents of the plant, previous studies have indicated that cryptotanshinone has potent activities against several kinds of cancers. For example, cryptotanshinone may be used as a signal transducer and activator of transcription 3 (STAT3) inhibitor to supress proliferation and growth of prostate cancer,20,21 human mucoepidermoid carcinoma22 and colorectal cancer,23 inducing endoplasmic reticulum stress-induced apoptosis in human hepatoma carcinoma HepG2 (ref. 24) and human breast cancer MCF7 cells.24,25 It is very interesting that cryptotanshinone could not only inhibit ER-positive breast cancer cell growth by suppressing estrogen receptor signaling,26 but also suppress androgen receptor-mediated growth in androgen dependent and castration resistant prostate cancer cells.27,28 However, it is little known about its roles in the ER-negative breast cancer.
Therefore, the purpose of this work was to investigate the effects of cryptotanshinone in ER-negative breast cancer cells. Previous study indicated that human breast cancer Bcap37 cells are ER-negative.29 Thus we used this cell line to evaluate the role of cryptotanshinone. We found that cryptotanshinone not only inhibited proliferation and migration of Bcap37 cells, but also induced mitochondrial-mediated apoptosis. Furthermore, we found that the cell apoptosis induced by cryptotanshinone involved ROS production, FOXO1 inhibition and several relative PI3K/AKT/mTOR signals. Our findings show that cryptotanshinone may be an efficient candidate for ER-negative breast cancer via activation of mitochondria-derived ROS/FOXO1 pathways.
2. Materials and methods
2.1. Materials
Cryptotanshinone (purity, more than 95%) was isolated and purified from the extracts of the root of S. miltiorrhiza Bunge by gradient counter-current chromatography method.15 Paclitaxel used as a positive contrast was purchased from National Institute for the Control of Pharmaceutical and Biological Products (NICPBP) (Beijing, China). Compounds were all dissolved in dimethyl sulfoxide (DMSO) and stored in −20 °C. 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) was purchased from Solarbio Science & Technology co., Ltd, Beijing, China. JC-1 Mitochondrial Membrane Potential Assay Kit was purchased from Cayman Chemical Company (CA, USA). Caspase inhibitor Z-VAD-FMK was purchased from Beyotime Biotechnology. BCA protein assay kit was purchased from Thermo Electron Co. MA, USA. AnnexinV-FITC-PI apoptosis kit was purchased from Biouniquer Technology Co., Ltd. Hoechst33342 was purchased from Invitrogen, CA, USA. ROS assay kit was purchased from Beyotime, China. Antibodies against ACTIN, PTEN, p-AKT (Ser 473), AKT, P-70S6K, p-P70S6K, FOXO1 and P27 were purchased from Cell Signalling Technology (Beverly, MA). Endo-G was purchased from SANTACRUZ Biotechnology Inc.2.2. Cell culture
The human breast cancer cell line Bcap37 was cultured in RPMI 1640 medium (Gibco, USA) supplemented with 10% NBCS (v/v) (Gibco, USA), 2 mM-glutamine, penicillin (100 U mL−1) and streptomycin (100 U mL−1) in humid condition of 37 °C containing 5% CO2.2.3. Scratch motility (wound-healing) assay
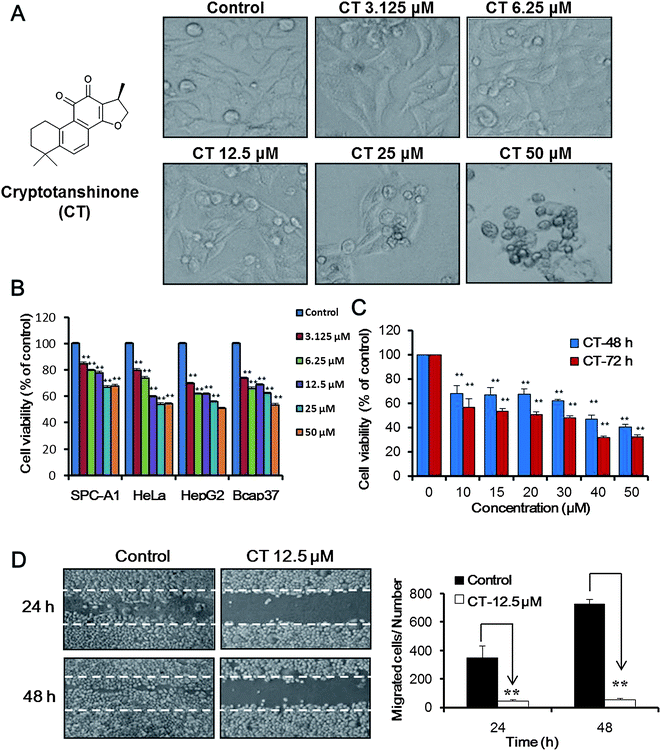
Bcap37 cells were firstly seeded in a 24-well plate to the density of 90%, scratched for would lines with an 10 μL pipette tip, washed with PBS for twice and marked the would line on the plate. Then the cells were incubated with cryptotanshinone at the concentration of 0, 12.5 μM, 25 μM, and 50 μM for 24 h or 48 h. Each treatment was set for three repeats. Finally, cell motility was measured by counting number of the cells that migrated beyond the reference line.302.4. Cell proliferation assay
Cell viability of Bcap37 cells was measured by MTT assay after treatment by tanshinones. Bcap37 cells (1 × 104) growing in logarithmic phase were firstly seeded in 96-well plate for 24 h. Then the cells were treated with cryptotanshinone at the concentrations range from 10 to 50 μM at a final volume of 200 μL each well. After incubation for 48 h, the function of cryptotanshinone was terminated by adding 20 μL MTT solution (5 mg mL−1) to each well and warmed in 37 °C for 4 h. Finally, the produced formazan crystals were dissolved in 100 μL DMSO and tested in a microplate reader at the wavelength of 570 nm.2.5. Apoptosis detection assays
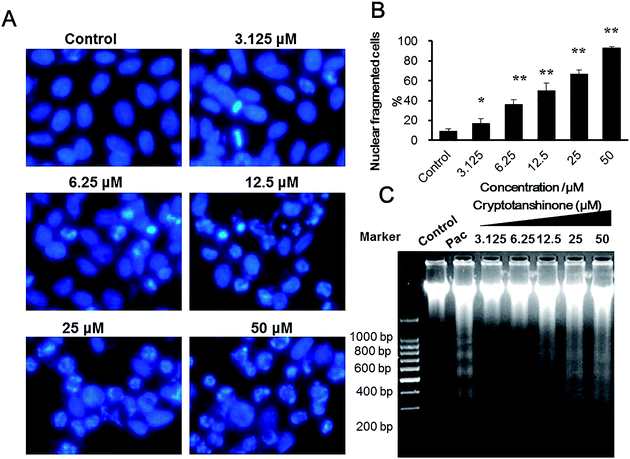
For DNA fragmentation assay, Bcap37 cells were seeded in a 6-well plate at the number of 1 × 105 cells per well, after 24 h, they were treated with cryptotanshinone for 48 h, and harvested for genomic DNA extraction using the phenol/chloroform/isopentanol method and then their DNA were run in a 1.5% agarose gel for DNA ladders. Finally, the image of gel was captured under the gel imaging instrument.
2.6. Cell cycle analysis
The ultra-pure water and analytically pure ethanol were prepared in 4 °C and −20 °C beforehand. Bcap37 cells seeded in 6-well plate were harvested in tubes at 2000 rpm for 5 min. The collected cells were suspended homogenizing distributed by 300 μL cold ultrapure water. Cell suspension was added to 700 μL pre-cold ethanol in each tube and mixed into uniform solution, kept in −20 °C for about 12 h, washed in PBS for at least once, centrifuged at 2000 rpm for 5 min. The cells were resuspended with 500 μL PI/RNase at a final concentration of 50 μg mL−1 for each tube, stained for at least 30 min and kept in 4 °C protected from light. Finally, the samples were analyzed on flow cytometer for cell cycle analysis. The data was analyzed using the ModiFit software for the analysis of cell cycle.2.7. Mitochondrial membrane potential assay
Bcap37 cells were seeded in 96-well plate as before, after 24 h, they were treated with cryptotanshinone at the concentration of 3.125 μM, 6.25 μM, 12.5 μM, 25 μM and 50 μM for 48 h, each treatment for three repeats. Cells were stained in situ with JC-1 alive at a final concentration of 2 μM, each well for 50–100 μL in 37 °C for about 30 min. Then they were washed with PBS for three times. Finally, the cells were imaged under an introverted fluorescence microscope.2.8. ROS detection assay
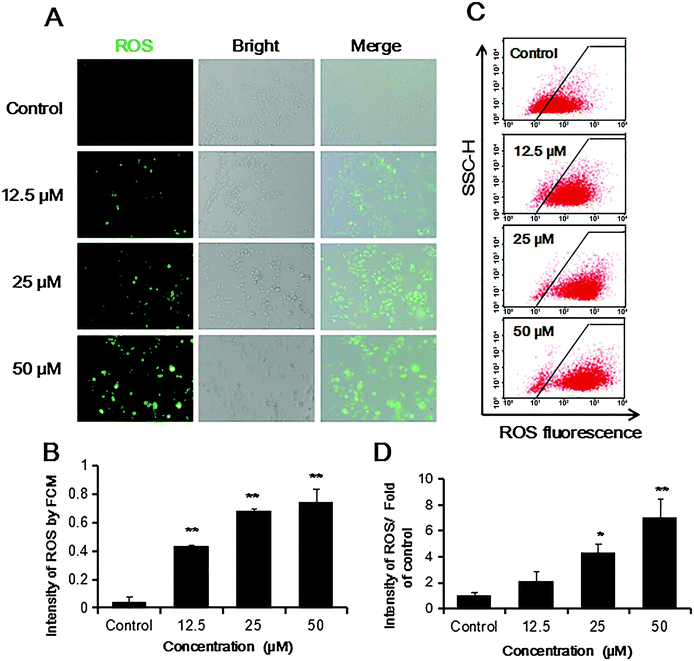
Bcap37 cells were seeded in 24-well plate, after 24 h, they were treated with or without cryptotanshinone at the concentration of 12.5 μM, 25 μM and 50 μM for 48 h, each treatment for three repeats. Cells were washed with PBS and incubated with ROS probe in serum free medium alive for 30 min in 37 °C according to the instruction of the kit. Then they were captured under an inverted fluorescence microscope at the wavelength of 488 nm. Additionally, Bcap37 cells were seeded on a 6-well plate and treated as above, harvested and incubated with ROS probes, the cells were analyzed by flow cytometry.2.9. Immunofluorescent staining
Bcap37 cells treated with cryptotanshinone were firstly washed twice with ice-cold PBS and fixed in 4% PFA for 30 min at room temperature, then they were washed in PBS for three times again. After being blocked in 5% BSA in PBS/Triton for 30 min, the cells were incubated with primary antibody by diluting in PBS/Triton at 4 °C overnight. Then the cells were rinsed three times in PBS for 5 min each, incubated with secondary antibody by diluting in PBS/Triton for 1 h in dark. At last, cells were stained with DAPI (5 μg mL−1) in PBS for 5 min and washed three times in PBS for 5 min each. Finally, the cells were captured under a confocal laser scanning microscope (Zeiss, LSM710).2.10. Western blot analysis
Proteins of cryptotanshinone treated Bcap37 cells for 48 h were harvested in lysis buffer on ice and centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min quickly. Supernatant was aspirated to another new tube, and quantified with BCA protein quantification kit (Thermo Scientific). The protein samples were loaded and run in SDS-PAGE gel, transformed to polyvinylidene fluoride (PVDF) membrane. The PVDF membrane was blocked in 5% non-fat milk for 1 h and incubated with primary antibody at 4 °C overnight, washed in PBST for 3 times, 10 min each time. Then it was incubated with secondary antibody for 1 h, and washed in PBST for 3 times, 10 min for each time. After the reaction of ECL, films were developed in dark and scanned.
000 rpm for 5 min quickly. Supernatant was aspirated to another new tube, and quantified with BCA protein quantification kit (Thermo Scientific). The protein samples were loaded and run in SDS-PAGE gel, transformed to polyvinylidene fluoride (PVDF) membrane. The PVDF membrane was blocked in 5% non-fat milk for 1 h and incubated with primary antibody at 4 °C overnight, washed in PBST for 3 times, 10 min each time. Then it was incubated with secondary antibody for 1 h, and washed in PBST for 3 times, 10 min for each time. After the reaction of ECL, films were developed in dark and scanned.
2.11. Statistical analysis
Data are expressed as means ± S.D. All experiments were repeated at least three times independently. Flow cytometry data of cell cycle were analyzed mainly by the software of ModiFit. Figures of western blot were analyzed by the software of image J. In all cases, p < 0.05 was considered significant.3. Results
3.1. Cryptotanshinone inhibits ER-negative breast cancer cell proliferation and migration
As shown in Fig. 1A, cryptotanshinone inhibited cell proliferation, induced cell shrinkage and condensed on morphology. Beside the widely effect on different kind of cell lines, MTT data (Fig. 1B and C) indicated that the inhibition effect of cryptotanshinone on Bcap37 cells were in dose- and time-dependent manner.Due to lack of estrogen receptor, Bcap37 cells showed stronger tendency of migration and invasion than other estrogen receptor positive breast cancers. Thus an in vitro wound healing assay30 was used to measure the cell motility under the treatment of cryptotanshinone. Above MTT data indicated the cryptotanshinone was cytotoxic against Bcap37 cells at the high concentration. Thus a relative low concentration 12.5 μM was used to evaluate the effects on migration of breast cancer cells. As illustrated in Fig. 1D, cryptotanshinone showed significant inhibition activity on wound healing (migration) of Bcap37 cells at the low concentration of 12.5 μM for 24 h or 48 h.
3.2. Cryptotanshinone arrests cell cycle at S phase
Cell cycle is a key regulator of cell survival and repair signals network, it has direct effects on multiple cell processes, including cell proliferation, DNA repair, apoptosis and cell migration.31 As described above, cryptotanshinone could inhibit cell proliferation and cell migration of Bcap37, thus cell cycle of Bcap37 was analyzed by flow cytometry. As shown in Fig. 2A and B, Bcap37 cells were reduced in G0/G1 phase, increased in S phase and showed no effect on G2/M phase, which indicated that the cells were arrested in S phase of mitosis cycle after treatment of different concentrations of cryptotanshinone for 48 h (Fig. 2B). Additionally, DNA in sub-G1 showed a gradually raising tendency following the increase of the concentration of cryptotanshinone (Fig. 2A and C), which suggested that there was DNA fragmentation in Bcap37 cells and the cells might begin to die through cell apoptosis pathway. This partially interpreted the inhibition on proliferation and migration of Bcap37 cells under the treatment of cryptotanshinone.3.3. Cryptotanshinone induced dose and time-dependent cell apoptosis
To clarify the formation of the DNA in sub-G1, the nuclear morphology of Bcap37 cells treated by cryptotanshinone was monitored. The results (Fig. 3A and B) indicated that there were an increasing number of nuclear condensed and fragmented cells with the concentration increase of cryptotanshinone. As is well known, one of the biochemical markers of apoptosis is DNA fragmentation into different fragments of 180 bp units.32 Thus the genomic DNA of Bcap37 cells was extracted and the DNA ladder assay was performed.As shown in Fig. 3C, there was significant amount of DNA fragments at 180, 360, 540 and 720 bp in Bcap37 cells treated by cryptotanshinone for 48 h at different concentrations from 3.125–50 μM. It was closely in agreement with the result of the increasing sub-G1 DNA detected from the cell cycle assay in cryptotanshinone treated Bcap37 cells at the concentration of 3.125–50 μM (Fig. 2C).
In addition, the cells treated by cryptotanshinone were analyzed by AnnexinV-FITC/PI assay on flow cytometry. The results (Fig. 4) indicated that cryptotanshinone induced the early apoptosis of Bcap37 cells in a dose- (Fig. 4A and B) and time- (Fig. 4C and D) dependent manner. When the Bcap37 cells were treated at the concentration of 25 μM for 48 h, a proportion of about 18% were induced to be early apoptotic cells. To explore the involved apoptotic pathway in Bcap37 cells, the optimum concentration of cryptotanshinone at 25 μM was selected for the following assays.
3.4. Cryptotanshinone decreased mitochondrial membrane potential
Since mitochondria are cellular organelles that are sensitive to apoptosis, we applied the mitochondria membrane potential detecting fluorescence probe JC1 in this case. As shown in Fig. 5A and B, it was apparent that mitochondria membrane potential was gradually decreased with the increase of the concentration of cryptotanshinone from 6.25 μM to 50 μM. Typically, the mitochondria membrane potential of cells showed distinct decrease by 20–60% under the concentration of cryptotanshinone from 12.5 μM to 50 μM compared with the intensity in control cells. This implied that cryptotanshinone induced Bcap37 cells apoptosis through mitochondria dependent intrinsic pathway.3.5. Cryptotanshinone induced caspase-dependent and independent apoptosis in Bcap37 cells
In most of the cases, cell apoptosis mainly involved activation of several caspases including caspase initiators and caspase executioners.33 As shown in Fig. 5C, Bcap37 cells treated by cryptotanshinone for 48 h showed apparent nuclear fragmentations and the increased expression of cleaved caspase-3 which is an active form of caspase executioner cleaved from caspase-3. Thus the result indicated that the apoptosis induced by cryptotanshinone underwent a caspase-dependent process. To confirm this, we measured cell apoptosis after the pre-treatment with or without caspase inhibitors for 2 h before co-culturing Bcap37 cells with cryptotanshinone. However, we found that the apoptosis was only slightly rescued when treated with cryptotanshinone together with the caspase inhibitor (Z-DEVD-fmk) in Bcap37 cells, compared with cryptotanshinone only treated cells (Fig. 5D and E). It implied that a caspase-independent pathway might also be involved in the cryptotanshinone-induced cell death.Besides caspase-dependent apoptosis, cell death also goes in the caspase-independent ways33 by activating several signals such as apoptosis-inducing factor (AIF)34 and nuclear encoded protein endonuclease G (EndoG).35 As is well known, EndoG is a mitochondria protein that can lead to DNA fragmentation directly and represents a caspase-independent pathway in cell apoptosis.35 In our case, the mitochondrial protein EndoG was shown to gradually translocate from mitochondria to nucleus according to the progress of cell apoptosis (Fig. 5F). Additionally, we quantified the expression of EndoG at the protein level and found that the expression level of EndoG was up-regulated especially under the treatment of high concentration of 12.5 and 25 μM of cryptotanshinone (Fig. 5G).
3.6. Cryptotanshinone induced apoptosis involved ROS production, FOXO1 inhibition and PI3K/AKT signalling pathways
Recently, reactive oxygen species (ROS)-targeting strategy has been thought to be a promising radical therapeutic approach for cancer cells. Chemically, ROS is a collective term that describes the chemical species that are formed upon incomplete reduction of oxygen and includes the superoxide anion (O2−), hydrogen peroxide (H2O2) and the hydroxyl radical (HO˙).36A moderate increase in ROS can promote cell proliferation, cell differentiation and protect cells from apoptosis, whereas excessive amounts of ROS can bring about oxidative damage to lipids, protein and DNA,37 the disruption of the mitochondria membrane potential and the release of cytochrome C by oxidation of mitochondrial pores, which subsequently induces cell apoptotic death.38,39 There are numbers of anti-cancer compounds that were found to induce cell death through ROS-mediated mechanism. For example, accumulation of hydrogen peroxide is an early and crucial step for paclitaxel-induced cancer cell death both in vitro and in vivo.40
However, cancer cells can express much more antioxidants such as FOXO1 to resist the cytotoxic stress.41 FOXO1 protein is one of the transcription factors that are implicated in cell cycle, DNA damage, cell proliferation and apoptosis. Oxidative stress could induce nuclear translocation of FOXO proteins, thus FOXO proteins could induce expressions of genes involved in oxidative stress resistance, cell cycle arrest and apoptosis.31–33 Screening some new ROS generators with lower antioxidant levels such as arsenic trioxide (As2O3)42 and the natural product piperlongumine43 are still very important for current drug development.
In this case, ROS production in Bcap37 cells was highly increased in a concentration dependent way (Fig. 6), which was in accordance with the known results that cryptotanshinone induced apoptosis in other tumor cells such as rhabdomyosarcoma (Rh30), prostate carcinoma (DU145) and HepG2 cells.44–46
It has been known that ROS can also act as second messengers regulating cell signaling pathways and also induce cell apoptosis or senescence when it is accumulated at high level.47,48 In our works, the PI3K/AKT pathway was found to be activated by treatment of cryptotanshinone (Fig. 7A). In addition, the tumor suppressor PTEN was also inhibited, which directly led to the increase of the p-AKT (Ser473) even at the low concentration of 6.25 μM.
The downstream transcription factor FOXO1, an antioxidant player and an inhibitor of cell apoptosis,48,49 was responsively decreased in Bcap37 cells (Fig. 7A, B and D). Additionally, phosphorylation of the substrate (P-P70S6K) of mTOR pathway was found to be increased (Fig. 7E and F), which contributed to the high level of ROS.
4. Discussion
Diterpenoid tanshinones have attracted particular attention from medicinal chemists and clinicians, because tanshinones possess a variety of pharmacological activities such as antibacterial, antioxidant, anti-inflammatory, and antineoplastic.19 Besides three tanshinones including tanshinone IIA, tanshinone I, and cryptotanshinone as the major constituents of the plant, there are total 40 tanshinones to be isolated from the plant.17,19 Among the tanshinones, cryptotanshinone has been found to be more efficient or sensible to several human cancer cells than other tanshinones.In the present study, we found that cryptotanshinone could inhibit ER-negative breast cancer Bcap37 cells proliferation and migration (Fig. 1) and induce shrinkage on cell morphology (Fig. 1A), cell cycle arrest in S phase and sub-G1 (Fig. 2). Apoptosis, or programmed cell death, is central to the development and homeostasis of metazoans.33,50 The morphological changes of apoptosis include membrane blebbing, cell shrinkage, chromatin condensation, and formation of apoptotic bodies.51 We observed these characteristics of apoptotic morphological changes including nuclear condensation or fragmentation and DNA fragmentation after cryptotanshinone treatment in Bcap37 cells (Fig. 3). Annexin V/PI assay which allows us to quantify the apoptotic cells, showed that cryptotanshinone induced early apoptosis on Bcap37 cells in a dose- and time-dependent way (Fig. 4).
Mitochondria play key roles in activating apoptosis in mammalian cells.52 We found that there was a significant decrease of mitochondrial membrane potential in Bcap37 cells induced by cryptotanshinone (Fig. 5A and B). Then it was also found that the active apoptotic protein cleaved-caspase 3 highly expressed especially in the nuclear-fragmented cells induced by cryptotanshinone (Fig. 5C and D). This suggested that cryptotanshinone induced a caspase-mediated apoptosis.
However, caspases inhibitor could not arrest completely the cryptotanshinone-induced apoptosis (Fig. 5D and E). This implied that there was a caspase-independent apoptotic pathway. There are several signals involving the activation of caspase-independent apoptosis process, such as AIF and EndoG.34,35 Here we found the protein EndoG gradually translocated to nucleus (Fig. 5F) and highly expressed especially under high stress of cryptotanshinone (Fig. 5G). In this case, EndoG released independent of the activation of caspases. It indicated that cryptotanshinone might meanwhile trigger the caspase-independent apoptotic pathway together with caspase-dependent apoptotic pathway when under high concentration of cryptotanshinone in Bcap37 cells.
ROS is one of the most important signals triggering apoptosis.48 As shown in Fig. 6, cryptotanshinone induce high level of ROS in a concentration dependent way in Bcap37 cells. It has been known that mitochondria is the source and also the target of ROS, while excessive ROS might disrupt mitochondria membrane potential48,53 and induce apoptosis.48,54 In this case, Bcap37 cells showed low mitochondrial membrane potential and fell in caspase-independent and dependent apoptosis under high level of ROS (Fig. 5).49
ROS is also an important second messenger to activate a series of cell signalling pathways such as ERK pathway, SAPK pathways, PKC pathway and PI3K/AKT pathway, and so on.50,55,56 In the current study, the PI3K/AKT pathway (Fig. 7) was found activated by cryptotanshinone and the downstream signals were correspondingly regulated.
FOXO1 is a transcriptional factor and acts as an antioxidant which can inhibit the production of ROS.57 Previous study58 indicated that FOXO1 may promote wound healing and prevention of oxidative stress. In this work, FOXO1 was inhibited evidently, resulting in increasing ROS accumulation (Fig. 6) and the inhibition of wound healing (Fig. 1D). In addition, one of the substrates of mTOR pathway phosphorylated-P70S6K was also activated. Additionally, activation of the PI-3K/AKT pathway and the nucleus translocation of FOXO1 from the cytoplasm might increase the level of the key cell division regulator P27, which contributed to arrest of cell cycle at S phase in Bcap37 cells and was responsible for cell proliferation inhibition.59
Therefore, cryptotanshinone induced cell apoptosis, inhibited cell proliferation and cell migration through ROS mediated PI3K/AKT/mTOR pathway in breast cancer Bcap37.
5. Conclusions
In summary, as proposed in Fig. 8, this work provided new clues to understand the mechanism of cryptotanshinone induced apoptosis of ER-negative breast cancers. Although we still need do many works to know its mechanism in vivo in future, our present study demonstrated that cryptotanshinone was an efficient ROS generator in vitro, and could inhibit cell proliferation and migration, induce apoptotic cell death in the ER-negative breast cancer cells Bcap37 by inhibiting the antioxidant FOXO1 through the activation of the PI3K/AKT/mTOR signaling pathway. Thus, cryptotanshinone might be a promising candidate in the treatment of ER-negative breast cancers by inducing apoptosis and inhibiting cell migration through the ROS-mediated PI3K/AKT/mTOR pathways. To the best of our knowledge, this is the first report to demonstrate the potential anti-cancer efficiency of cryptotanshinone on ER-negative breast cancer through the ROS-mediated PI3K/AKT/mTOR signalling pathways.Acknowledgements
The authors apologize to colleges whose work could not be cited owing to lack of space. This work was supported in part by the National Natural Science Foundation of China (Grant No. 20972136, 21272209).References
- R. Siegel, E. Ward, O. Brawley and A. Jemal, Ca-Cancer J. Clin., 2011, 61, 212–236 CrossRef PubMed.
- R. Siegel, D. Naishadham and A. Jemal, Ca-Cancer J. Clin., 2013, 63, 11–30 CrossRef PubMed.
- A. Brodie and G. Sabnis, Clin. Cancer Res., 2011, 17, 4208–4213 CrossRef CAS PubMed.
- E. R. Simpson, J. Steroid Biochem. Mol. Biol., 2003, 86, 225–230 CrossRef CAS PubMed.
- J. D. Yager and N. E. Davidson, N. Engl. J. Med., 2006, 354, 270–282 CrossRef CAS PubMed.
- Y. Tsuchiya, M. Nakajima and T. Yokoi, Cancer Lett., 2005, 227, 115–124 CrossRef CAS PubMed.
- I. E. Smith and M. Dowsett, N. Engl. J. Med., 2003, 348, 2431–2442 CrossRef CAS PubMed.
- S.-X. Lin, J. Chen, M. Mazumdar, D. Poirier, C. Wang, A. Azzi and M. Zhou, Nat. Rev. Endocrinol., 2010, 6, 485–493 CrossRef CAS PubMed.
- A. Howell and M. Dowsett, Br. Med. J., 1997, 315, 863–866 CrossRef CAS PubMed.
- J. N. A. G. M. Cragg, J. Nat. Prod., 2012, 75, 311–335 CrossRef PubMed.
- J. W. H. L. A. J. C. Vederas, Science, 2009, 325, 161–165 CrossRef PubMed.
- F. E. K. A. G. T. Carter, Nat. Rev. Drug Discovery, 2005, 4, 206–220 CrossRef PubMed.
- J. Meng, Z. Yang, J. Liang, H. Zhou and S. Wu, J. Chromatogr. A, 2014, 1323, 73–81 CrossRef CAS PubMed.
- J. Meng, Z. Yang, J. Liang, M. Guo and S. Wu, J. Chromatogr. A, 2014, 1327, 27–38 CrossRef CAS PubMed.
- S. Wu, D. Wu, J. Liang and A. Berthod, J. Sep. Sci., 2012, 35, 964–976 CrossRef CAS PubMed.
- D. Wu, X. Jiang and S. Wu, J. Sep. Sci., 2010, 33, 67–73 CrossRef CAS PubMed.
- Y. G. Li, L. Song, M. Liu, H. Zhi Bi and Z. T. Wang, J. Chromatogr. A, 2009, 1216, 1941–1953 CrossRef CAS PubMed.
- L. M. Zhou, Z. Zuo and M. S. S. Chow, J. Clin. Pharmacol., 2005, 45, 1345–1359 CrossRef CAS PubMed.
- X. H. Wang, S. L. Morris-Natschke and K. H. Lee, Med. Res. Rev., 2007, 27, 133–148 CrossRef CAS PubMed.
- D. S. Shin, H. N. Kim, K. D. Shin, Y. J. Yoon, S.-J. Kim, D. C. Han and B.-M. Kwon, Cancer Res., 2009, 69, 193–202 CrossRef CAS PubMed.
- K. Sung-Hoon, Korean J. Orient. Physiol. Pathol., 2012, 26, 437–440 Search PubMed.
- H. J. Yu, C. Park, S.-J. Kim, N. P. Cho and S. D. Cho, Pharmacogn. Mag., 2014, 10, 622–629 CrossRef PubMed.
- W. Li, S. M. Saud, M. R. Young, N. H. Colburn and B. Hua, Mol. Cell. Biochem., 2015, 406, 63–73 CrossRef CAS PubMed.
- I. J. Park, M. J. Kim, O. J. Park, W. Choe, I. Kang, S. S. Kim and J. Ha, Apoptosis, 2012, 17, 248–257 CrossRef CAS PubMed.
- Y. F. Zhang, M. Zhang, X. L. Huang, Y. J. Fu, Y. H. Jiang, L. L. Bao, Y. Maimaitiyiming, G. J. Zhang, Q. Q. Wang and H. Naranmandura, Metallomics, 2015, 7, 165–173 RSC.
- S. Li, H. Wang, L. Hong, W. Liu, F. Huang, J. Wang, P. Wang, X. Zhang and J. Zhou, Cancer Biol. Ther., 2015, 16, 176–184 CrossRef CAS PubMed.
- D. Xu, T.-H. Lin, S. Li, J. Da, X.-Q. Wen, J. Ding, C. Chang and S. Yeh, Cancer Lett., 2012, 316, 11–22 CrossRef CAS PubMed.
- Y. Zhang, S.-H. Won, C. Jiang, H.-J. Lee, S.-J. Jeong, E.-O. Lee, J. Zhang, M. Ye, S.-H. Kim and J. Lu, Pharm. Res., 2012, 29, 1595–1608 CrossRef CAS PubMed.
- M. Sui, Y. Huang, B. H. Park, N. E. Davidson and W. Fan, Cancer Res., 2007, 67, 5337–5344 CrossRef CAS PubMed.
- L. G. Rodriguez, X. Wu and J.-L. Guan, Methods Mol. Biol., 2005, 294, 23–29 Search PubMed.
- M. Boehm and E. G. Nabel, Circulation, 2001, 103, 2879–2881 CrossRef CAS PubMed.
- A. H. Wyllie, J. F. Kerr and A. R. Currie, Int. Rev. Cytol., 1980, 68, 251–306 CAS.
- S. J. Riedl and Y. Shi, Nat. Rev. Mol. Cell Biol., 2004, 5, 897–907 CrossRef CAS PubMed.
- L. Yang, X. Liu, Z. Lu, J. Y.-W. Chan, L. Zhou, K.-P. Fung, P. Wu and S. Wu, Cancer Lett., 2010, 298, 128–138 CrossRef CAS PubMed.
- L. Y. Li, X. Luo and X. Wang, Nature, 2001, 412, 95–99 CrossRef CAS PubMed.
- B. D'Autreaux and M. B. Toledano, Nat. Rev. Mol. Cell Biol., 2007, 8, 813–824 CrossRef PubMed.
- D. Trachootham, J. Alexandre and P. Huang, Nat. Rev. Drug Discovery, 2009, 8, 579–591 CrossRef CAS PubMed.
- J. Watson, Open Biol., 2013, 3, 120144 CrossRef PubMed.
- C. Wang and R. J. Youle, Annu. Rev. Genet., 2009, 43, 95–118 CrossRef CAS PubMed.
- J. Alexandre, F. Batteux, C. Nicco, C. Chereau, A. Laurent, L. Guillevin, B. Weill and F. Goldwasser, Int. J. Cancer, 2006, 119, 41–48 CrossRef CAS PubMed.
- T. Goto, M. Takano, J. Hirata and H. Tsuda, Br. J. Cancer, 2008, 98, 1068–1075 CrossRef CAS PubMed.
- J. Lu, E.-H. Chew and A. Holmgren, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 12288–12293 CrossRef CAS PubMed.
- L. Raj, T. Ide, A. U. Gurkar, M. Foley, M. Schenone, X. Li, N. J. Tolliday, T. R. Golub, S. A. Carr, A. F. Shamji, A. M. Stern, A. Mandinova, S. L. Schreiber and S. W. Lee, Nature, 2011, 475, 231–234 CrossRef CAS PubMed.
- W. Y. W. Lee, K. W. K. Liu and J. H. K. Yeung, Cancer Lett., 2009, 285, 46–57 CrossRef CAS PubMed.
- I.-J. Park, M.-J. Kim, O. J. Park, M. G. Park, W. Choe, I. Kang, S.-S. Kim and J. Ha, Cancer Lett., 2010, 298, 88–98 CrossRef CAS PubMed.
- W. Chen, L. Liu, Y. Luo, Y. Odaka, S. Awate, H. Zhou, T. Shen, S. Zheng, Y. Lu and S. Huang, Cancer Prev. Res., 2012, 5, 778–787 CrossRef CAS PubMed.
- J. E. Le Belle, N. M. Orozco, A. A. Paucar, J. P. Saxe, J. Mottahedeh, A. D. Pyle, H. Wu and H. I. Kornblum, Cell Stem Cell, 2011, 8, 59–71 CrossRef CAS PubMed.
- H. U. Simon, A. Haj-Yehia and F. Levi-Schaffer, Apoptosis, 2000, 5, 415–418 CrossRef CAS PubMed.
- V. J. Thannickal and B. L. Fanburg, Am. J. Physiol.: Lung Cell. Mol. Physiol., 2000, 279, L1005–L1028 CAS.
- P. D. Ray, B.-W. Huang and Y. Tsuji, Cell. Signalling, 2012, 24, 981–990 CrossRef CAS PubMed.
- S. Rello, J. C. Stockert, V. Moreno, A. Gamez, M. Pacheco, A. Juarranz, M. Canete and A. Villanueva, Apoptosis, 2005, 10, 201–208 CrossRef CAS PubMed.
- D. R. Green and J. C. Reed, Science, 1998, 281, 1309–1312 CrossRef CAS PubMed.
- G.-Y. Li, K. H. Jung, H. Lee, M. K. Son, J. Seo, S.-W. Hong, Y. Jeong, S. Hong and S.-S. Hong, Cancer Lett., 2013, 329, 59–67 CrossRef CAS PubMed.
- S. Rakshit, L. Mandal, B. C. Pal, J. Bagchi, N. Biswas, J. Chaudhuri, A. A. Chowdhury, A. Manna, U. Chaudhuri, A. Konar, T. Mukherjee, P. Jaisankar and S. Bandyopadhyay, Biochem. Pharmacol., 2010, 80, 1662–1675 CrossRef CAS PubMed.
- M. Zamkova, N. Khromova, B. P. Kopnin and P. Kopnin, Cell Cycle, 2013, 12, 826–836 CrossRef CAS PubMed.
- J. L. Martindale and N. J. Holbrook, J. Cell. Physiol., 2002, 192, 1–15 CrossRef CAS PubMed.
- Y. Akasaki, O. Alvarez-Garcia, M. Saito, B. Carames, Y. Iwamoto and M. K. Lotz, Arthritis Rheumatol., 2014, 66, 3349–3358 CrossRef CAS PubMed.
- B. Ponugoti, F. Xu, C. Zhang, C. Tian, S. Pacios and D. T. Graves, J. Cell Biol., 2013, 203, 327–343 CrossRef CAS PubMed.
- M. B. Moller, Leuk. Lymphoma, 2000, 39, 19–27 CAS.
| This journal is © The Royal Society of Chemistry 2016 |