Preparation of DyVO4/WO3 heterojunction plate array films with enhanced photoelectrochemical activity†
Abstract
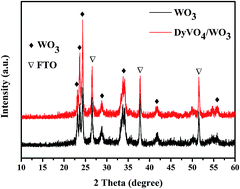
In this work, DyVO4/WO3 heterojunction plate arrays were first fabricated on FTO using a hydrothermal method for WO3 vertical plate arrays and a dipping–annealing process for the deposition of DyVO4 nanoparticles. The samples were characterized by various techniques, including X-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Photoelectrochemical activities were investigated by linear sweep voltammetry (LSV) and incident photon to current conversion efficiency (IPCE). The DyVO4/WO3 heterojunction electrode exhibited a maximum photocurrent density of 0.78 mA cm−2 at +1.2 V (vs. Ag/AgCl), while the photocurrent density of pure WO3 was just 0.49 mA cm−2 under illumination. And the highest IPCE value increased from 27.4% to 54.1% after the DyVO4 nanoparticle deposition. The enhanced PEC performance was attributed to the longer electron lifetime, increased carrier density and high charge separation efficiency at the interface of heterojunction, which were confirmed by electrochemical impedance spectroscopy (EIS) and Mott–Schottky analysis. The study demonstrates that metal orthovanadates may be good heterojunction candidates to couple with WO3 to provide promising photoanodes for water splitting.

 Please wait while we load your content...
Please wait while we load your content...