A new magnesium-containing aluminophosphate with a zeolite-like structure†
Abstract
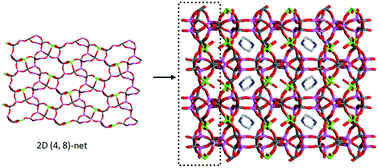
A novel Mg-containing aluminophosphate |C4H12N2|[Mg2Al6(PO4)8(H2O)4] (JU96) has been hydrothermally synthesized using piperazine as a structure-directing agent. Its framework, constructed by the connection of AlO4/MgO4(H2O)2 polyhedra and PO4 tetrahedra, exhibits a new 4-connected zeolite-like topology with intersecting 8-ring channels. Such a framework contains unique [44·66·86] cages that are occupied by diprotonated piperazine cations. Study shows that the presence of Mg atoms and the pH value of the reaction gel have a vital effect on the formation of JU96.

 Please wait while we load your content...
Please wait while we load your content...