Facile fabrication of reduced graphene oxide covered ZnCo2O4 porous nanowire array hierarchical structure on Ni-foam as a high performance anode for a lithium-ion battery†
Yujue Wanga,
Yongzhi Zhangb,
Junke Ouc,
Qian Zhaod,
Mei Liaoa and
Dan Xiao*ad
aCollege of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu 610064, China. E-mail: xiaodan@scu.edu.cn; Fax: +86-28-85412907; Tel: +86-28-85416218
bInstitute of New Energy and Low-Carbon Technology, Sichuan University, No. 24 South Section 1, Yihuan Road, Chengdu 610065, China. Fax: +86-28-6213-8325; Tel: +86-28-6213-8375
cSchool of Basic Medical Sciences & Nursing, Chengdu University, Shiling Town, Chengdu 610106, China. E-mail: ojk0001@163.com
dCollege of Chemical Engineering, Sichuan University, 29 Wangjiang Road, Chengdu 610064, China. E-mail: xiaodan@scu.edu.cn; Fax: +86-28-85415029; Tel: +86-28-85416029
First published on 7th December 2015
Abstract
We successfully prepared a nanostructure from ZnCo2O4 nanowire arrays directly grown on a Ni-foam substrate, with a surface decorated with reduced graphene oxide (rGO), through a very simple and cost-effective two-step facile fabrication using a hydrothermal method together with calcination treatment, followed by electro-deposition. It is shown that there are plenty of pores distributed along each of the nanowires, providing a large specific surface area. We also observe that the rGO thin flakes have indeed covered the ZnCo2O4 nanowires. This binder-free hierarchical composite on a Ni substrate was further used as an anode for lithium ion batteries. It displays a largely improved electrochemical performance, with a high capacity, excellent cycling stability and good rate capability, compared to both the anode without rGO covering and the anode with binder and conductive additive. It can retain a high discharge capacity of 1208 mA h g−1 after 100 cycles at the rate of 0.1 A g−1, and in the mean time, it can also provide a high capacity of 1032 mA h g−1 after 100 cycles even at a high rate of 0.5 A g−1. In this paper we prove that our rGO covered ZnCo2O4 nanowire array hierarchical structure supported on a Ni-foam substrate is a promising anode material for high performance lithium storage devices.
Introduction
Nowadays, rechargeable lithium ion batteries (LIBs) have become the regnant energy source and are widely used, and the desire for the energy materials applied in electronic devices and electric vehicles is turning increasingly intense. The most commonly utilized anode material for commercial LIBs, graphite, can hardly meet these needs because its theoretical specific capacity is quite low (only 372 mA h g−1), and moreover, its rate capability is relatively poor.1–3 With this in mind, more extensive attention has been directed to researching and developing advanced electrode materials of high performance, low price and benignity to the environment for LIBs which could be a substitute for graphite electrodes.1–8As a result, people have widely exploited transition metal oxides as anode materials for LIBs, for instance, TiO2, Fe2O3, Co3O4, SnO2, etc., because they can have high reversible capacities of about 500–1000 mA h g−1 (ref. 6 and 9–11) and can easily be made on the nanoscale through a solution route.12 ZnCo2O4 is among one of the most promising electrode materials13–15 as it has a large capacity, and is of less toxicity and lower cost than the widely studied Co3O4.16,17 However, its poor conductivity and evident volume change during the processes of Li+ intercalation and de-intercalation could give rise to the rupturing of electrode films in the process of electrical isolation, further resulting in rapid capacity fading. These disadvantages represent a big barrier in the application in LIBs.18
Nowadays, graphene and graphene oxide (GO) are the most promising materials,19 having applications in the fields of physics,20 chemistry21 and materials science.22 The conductivity of highly reduced monolayers of GO ranges between 0.05 and 2 S cm−1,23 which together with its water solubility, makes it promising for the construction of nanocomposites with other materials via a simple aqueous electro-deposition method for electrochemical usage with improved performance.24–26
Therefore, we prepared rGO covered ZnCo2O4 nanowire array nanostructures directly on Ni-foam using a simple two-step fabrication through a hydrothermal method together with annealing treatment followed by electro-deposition. It possesses several advantages: (1) both the hydrothermal method and electro-deposition are low cost, and of easy operation and effectivity; (2) not only can the rGO shell improve conductivity, but it can also cover the ZnCo2O4 arrays and prevent them from peeling off and crushing, thus enhancing the rate capability and retarding the capacity fading; (3) these 3D hierarchical structures can utilize not only their unique size and shape for electron and Li+ transport in the solid state, but also their large surface area for increased active material–electrolyte contact;27–31 (4) moreover, the absence of binder and conductive additive could increase electrolyte accessibility to the active material, so we can achieve a higher performance in anode material for LIBs.32 With these unique merits, our 3D hierarchical structure of rGO covered ZnCo2O4 nanowire arrays displays improved rate capability and cycling stability.
Experimental
Materials preparation
Material characterization
Several different techniques were applied to characterize the samples we prepared. The crystal structure of the samples was characterized using an X-ray diffractometer (XRD; Tongda TD-3500, Liaoning, China) with Cu Kα radiation (λ = 1.5418 Å) running at 30.0 kV and 20.0 mA. Scanning electron microscopy (SEM) was performed using a Hitachi S4800 scanning electron microscope (Tokyo, Japan) to analyze the morphology of the samples. Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) were performed using an FEI Tecnai G2 20 TEM (Hillsboro, OR, USA) operating at 200 kV. Inductively coupled plasma atomic emission spectroscopy (ICP-AES) was performed using an IRIS Advantage (ThermoElemental, USA). Carbon content was determined using a EuroEA3000 Analyzer (Leeman, USA). The specific surface area and pore size distribution were obtained from the results of nitrogen (N2) adsorption and desorption using an automated gas sorption system (Quantachrome NOVA 1000e apparatus) using Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) techniques. Raman measurements were carried out using a LabRAM HR800 spectrometer (HORIBA Jobin Yvon, France). Thermogravimetric analysis (TGA) was conducted using a Henven HCT-2 thermal analyzer (Beijing, China). X-ray photoelectron spectroscopy (XPS) characterization was performed using a Kratos XSAM800 spectrometer (Manchester, UK), with a MgKα X-ray (1253.6 eV) excitation source running at 15 kV, a hemispherical electron energy analyzer and a multichannel detector.Electrochemical measurements
The Ni-foam covered with ZnCo2O4 was cut into many small pieces. The loading density of the as-prepared samples was around 1.05 to 1.76 mg cm−2, and each piece of the samples was used directly as a working electrode without any addition of acetylene black or binder. In comparison, we also collected the ZNW powder (ZP) scraped from the Ni-foam and formed it into working electrodes. The electrodes were prepared through mixing 80 wt% active material, 10 wt% polyvinylidene fluoride (PVDF) and 10 wt% acetylene black (AB) before dispersing this in N-methylpyrrolidone (NMP) to form a uniform slurry. Then the slurry was spread on copper foil evenly and vacuum dried at 100 °C. Samples were assembled into CR2032 coin-type cells in a glovebox (Delix LS800S, Sichuan, China) filled with Ar, using the ZNWG, ZNW on Ni-foam (ZNWG–Ni and ZNW–Ni) and ZP as anode materials, Li-metal foil as both counter and reference electrodes, a piece of Celgard 2300 microporous film as a separator, and 1.0 M LiPF6 solution in ethylene carbonate (EC) and dimethyl carbonate (DMC) (volumetric ratio EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC = 1
DMC = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the electrolyte. Before measurement the cell was aged for 24 h to make sure that the electrolyte fully permeated into the electrodes.
1) as the electrolyte. Before measurement the cell was aged for 24 h to make sure that the electrolyte fully permeated into the electrodes.
Then the electrochemical measurements were carried out and the coin-type half cells were cycled over a voltage range of 0.01–3 V (vs. Li+/Li) with different current densities using a multichannel battery measurement system (Land, Hubei, China) at room temperature. The electrochemical impedance spectroscopy (EIS) and cyclic voltammetry (CV) were performed on an electrochemical workstation (Autolab PGSTAT 30/302, Eco Chemie B.V., Amsterdam, The Netherlands). The voltage range and scan rate of the CV measurements were 0.01–3 V and 0.1 mV s−1, respectively.
Results and discussion
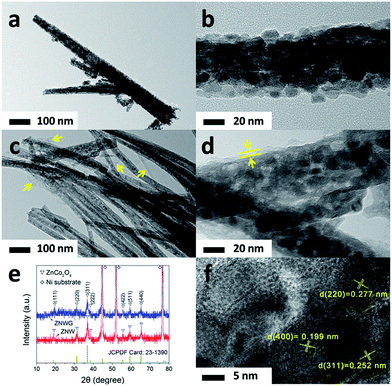
The morphologies of the as-prepared ZnCo2O4 nanowire arrays (ZNW) and rGO covered ZnCo2O4 nanowire arrays (ZNWG) on Ni-foam are shown in Fig. 1 and 2 following scanning electron microscopy (SEM, Fig. 1) and transmission electron microscopy (TEM, Fig. 2), respectively. Fig. 1a and c show low magnification SEM images of ZNW–Ni and ZNWG–Ni, respectively, with the contrast revealing that the ZnCo2O4 nanowires in ZNWG–Ni were covered in transparent thin films. Zoomed-in SEM images are shown in Fig. 1b and d, which give a clearer view of the 3D nanostructure of the as-prepared nanowires and the presence of rGO film. It is shown obviously that the diameter of the nanowires was about 100 nm and we can also observe plentiful pores distributed along the whole trunk. In the low magnification SEM image of ZNWG–Ni (Fig. 1c), we can see some dim areas likely to be covered by a layer of thin film, evidently distinct from ZNW–Ni, emphasized by yellow dashed lines showing the boundary of the covered and uncovered areas. We have a clearer view in the zoomed-in image (Fig. 1d) which displays obviously a transparent film, indicating that the ZnCo2O4 nanowires were covered with rGO film. For further confirmation that the rGO film really connectively decorated the whole surface of ZNW, a TEM investigation was carried out on both ZNW (Fig. 2a and b) and ZNWG (Fig. 2c and d). In contrast, we can see that on the surface of the ZnCo2O4 nanowires before electro-deposition, no film appeared (Fig. 2a and b), while the ZnCo2O4 nanowires after electro-deposition exhibited a layer of thin film (Fig. 2c and d), which further verified that rGO was indeed decorated on the surface of ZNW. With these, the fabrication process of ZNWG on Ni-foam is concluded and shown as a schematic illustration in Fig. 3.To determine the crystal structures of both the ZNW and ZNWG, X-ray diffraction (XRD) was carried out, with the patterns shown in Fig. 2e. Three strong diffraction peaks ascribed to the Ni-foam substrate were found in the pattern, which are marked with the symbol ◇, while another seven diffraction peaks marked with the symbol ▽, which correspond to those of cubic ZnCo2O4 with a spinel structure (JCPDF card 23-1390), were also displayed in the pattern. Additionally, no obvious extra diffraction peaks were detected, indicating that the product is of high purity.
ICP-AES analysis was conducted to obtain the metal ion content of the as-prepared ZNWG, to further confirm its pure phase. The results show that the Zn and Co content is 12.9 and 22.3 ppm, respectively, or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.92 as a molar ratio, which agrees well with the molar ratio of Zn to Co of 1
1.92 as a molar ratio, which agrees well with the molar ratio of Zn to Co of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in ZnCo2O4, further confirming the pure phase.
2 in ZnCo2O4, further confirming the pure phase.
Raman measurements were performed in order to further confirm the existence of rGO. Fig. 4 shows the Raman spectra for ZNWG and GO. The bands within the ranges of 250–700 and 880–1200 cm−1 belong to ZnCo2O4, while the ones in the ranges of 1200–1450 and 1500–1650 cm−1 are ascribed to the D band (K-point phonons of A1g symmetry) and G-band (E2g phonons of the C sp2 atoms) of GO, respectively, demonstrating the presence of GO in the composite. Compared to GO, the G band of GO in ZNWG shifts to a low frequency, indicating a significant removal of oxygen-containing groups in GO during electro-deposition,36 which is why we call it rGO. It is easily observed that ZNWG shows a lower intensity of D and G bands (ID/IG = 0.90) than GO freeze-dried from the GO dispersion (ID/IG = 0.99), which illustrates the electronic conjugation after reduction, leading to high electronic conductivity.37
In order to obtain the loading of rGO in ZNWG, thermogravimetric analysis (TGA) measurements were carried out for ZNWG and ZNW samples under an air atmosphere to acquire the carbon content (Fig. S1†). The slight weight loss at the very beginning could be ascribed to moisture in the samples. By comparing the two curves, it is easy to find that the content of rGO is 1.39%, which agrees with the result of the C , N, S test of 1.28%.
The chemical states of the as-prepared ZNWG sample were studied via X-ray photoelectron spectroscopy (XPS) in order to further confirm the purity of the sample, the appearance of rGO and the combination form of rGO and the ZnCo2O4 nanowires. Fig. 5a shows a full wide-scan spectrum of ZNWG. Characteristic peaks for Zn, Co, O, and C elements are easily observed. The binding energy from XPS analysis of the C 1s peak at 284.6 eV was used as the reference for calibration. Fig. 5b shows the high-resolution Zn 2p spectrum. Two peaks with binding energy values of 1021.480 and 1044.735 eV are ascribed to Zn 2p3/2 and Zn 2p1/2, indicating the Zn(II) oxidation state of ZnCo2O4.38,39 Fig. 5c shows the high-resolution Co 2p spectrum. Two strong peaks at 779.991 eV and 790.063 eV for Co 2p3/2 and Co 2p1/2 are observed, respectively, confirming the Co(III) oxidation state of ZnCo2O4.38,40 The O 1s peaks at 529.480 and 532.243 eV correspond to the oxygen species in ZnCo2O4, shown in Fig. 5d.15 From these, the XPS results in conjunction with the XRD data confirm the formation of ZnCo2O4 with a normal spinel structure. The C 1s spectrum in Fig. 5e possesses four main peaks that correspond to functional groups of carbonyl carbon in acetone/quinone, carboxylic carbon in –COOR (R = H and alkyl), ether/phenolic carbon in C–O–C and C–OH, and carbon atoms in the graphene planes.41 Compared to the XPS spectra of ZNW shown in Fig. S2,† no evident distinction is found in the corresponding high resolution Zn 2p, Co 2p and O 1s spectra, and no other forms of C are observed in the C 1s spectrum except for the ones mentioned above. These are all similar to previous works involving metal oxides physically coated with rGO or graphene.42,43 Furthermore, from the XRD patterns of ZNWG and ZNW in Fig. 2e, we can see that there is no obvious distinction between the two samples. No other peaks except for those of ZnCo2O4 have been observed. So it can be confirmed that rGO is not chemically bound but physically coated on the ZnCo2O4 nanowires during the electrochemical deposition process.
 | ||
| Fig. 5 XPS spectra of ZNWG: (a) survey spectrum and high resolution (b) Zn 2p, (c) Co 2p, (d) O 1s, and (e) C 1s spectra. | ||
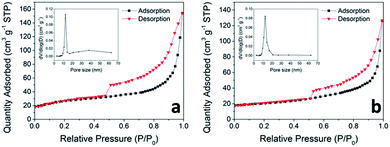
By reason of the plentiful pores observed in the SEM and TEM images, BET measurements of N2 adsorption and desorption were carried out to determine the specific surface area of the as-prepared ZNWG and ZNW, shown in Fig. 6. The as-prepared ZNWG sample displays a specific surface area of 76.32 m2 g−1, larger than that of ZNW, 68.13 m2 g−1. The appearance of rGO has increased the specific area to a certain degree, and the extent of electrolyte–material contact is improved due to the large specific surface area, bringing about a sufficient electrochemical reaction.44
The pore size distribution, deduced from desorption data and computed from the isotherm employing the BJH model (inset of Fig. 6a and b), shows that most of the pores in the nanowires are within the size range of 10 to 20 nm for both ZNWG and ZNW. This porous nanostructure can buffer the large volume change of the electrode during the repeated Li+ intercalation and de-intercalation.44
The typical electrochemical procedure for ZnCo2O4 concerning lithium intercalation and de-intercalation, as reported in previous literatures, is shown as follows:14,18
| ZnCo2O4 + 8Li+ + 8e− → Zn + 2Co + 4Li2O | (1) |
| Zn + Li+ + e− → LiZn | (2) |
| Zn + Li2O ↔ ZnO + 2Li+ + 2e− | (3) |
| 2Co + 2Li2O ↔ 2CoO + 4Li+ + 4e− | (4) |
2CoO + ⅔Li2O ↔ ⅔Co3O4 + ![[/]](https://www.rsc.org/images/entities/char_e12a.gif) Li+ + Li+ + ![[/]](https://www.rsc.org/images/entities/char_e12a.gif) e− e−
| (5) |
Electrochemical measurements such as cyclic voltammetry (CV) and galvanostatic discharging and charging have been conducted to test the electrochemical performance of the as-prepared ZNWG–Ni electrode. CV measurements were carried out at a scan rate of 0.1 mV s−1 and in a voltage range of 0.01–3 V. The first three CV curves for the ZNWG–Ni electrode are shown in Fig. 7g. In the first cathodic process, an obvious sharp irreversible reduction peak shows up at approximately 0.62 V, which is correlated to solid electrolyte interface (SEI) layer formation and the irreversible reduction of ZnCo2O4 to Zn and Co as shown in reaction (1).18,45 Then in the first anodic scanning process we observed two broad peaks at about 1.65 and 2.25 V, on account of the oxidation of Zn and Co to Zn2+ and Co2+, respectively, as displayed in reaction (3–5). From the 2nd cycle on, the reduction peak moves to a higher potential of approximately 0.9 V and transforms into a broader peak, which differs from the 1st cycle, indicating that the two processes involve different electrochemical reactions. The similar shape of the CV curves of the 2nd and 3rd cycle indicates the good reversibility of the electrode from the 2nd cycle on.
 | ||
| Fig. 7 (a, c and e) Galvanostatic discharge and charge profiles for ZNWG–Ni (a), ZNW–Ni (c) and ZP (e) electrodes at a current density of 0.1 A g−1; (b, d and f) cycling performance of ZNWG–Ni (b), ZNW–Ni (d) and ZP (f) electrodes at a current density of 0.5 A g−1 with corresponding coulombic efficiencies; (g) first three CV curves of ZNWG–Ni electrode at a scan rate of 0.1 mV s−1; (h) rate capabilities of ZNWG–Ni, ZNW–Ni and ZP electrodes at different current densities. The corresponding active material loadings of the electrodes are shown in Table S1.† | ||
Fig. 7a, c and e show the discharge and charge curves for the first 100 cycles at a current density of 0.1 A g−1 over a voltage range of 0.01–3 V. In the first discharge procedure, the voltage suddenly dropped to approximately 1 V, which was due to the conversion reaction as shown in reaction (1), and this is in accordance with the CV results. Then there exists a long plateau at about 1 V, followed by a slope beneath 1 V, which has been fully discussed in previous papers.15,18,45 From the 2nd cycle on, the long discharging plateau changed into a slope, which agreed well with some previous reports,18,46 indicating that a stable solid electrolyte interface (SEI) formed during the first cycle.47 The initial discharge and charge capacities for the ZNWG–Ni electrode were 1946 and 1371 mA h g−1, respectively, as shown in Fig. 7a. The high initial discharge capacity might be on account of the large surface area. Due to the formation of an SEI and Li2O during the reduction of metal oxide to metal,48–50 the irreversible capacity of the first cycle lost 29.5%. The discharge and charge capacities and relative coulombic efficiency of ZNWG–Ni over 100 cycles under 0.5 A g−1 are shown in Fig. 7b. It can be seen that a very high reversible discharge capacity of 1170 mA h g−1 was attained, and even after 100 cycles, it still remained at 1032 mA h g−1, which indicates the excellent cycling performance of the ZNWG–Ni electrode. The increase in capacity from the 20th to the 50th cycle was most likely to be due to the result of material activation due to the full contact with the electrolyte and the reversible growth of a polymeric gel-like film on account of kinetically activated electrolyte degradation.51,52 Additionally, in the 2nd cycle, the coulombic efficiency rose from the 65.5% of the 1st cycle to 94%, and kept over 96% after the 10th cycle, indicating the good reversibility of the ZNWG–Ni electrode.
For comparison, the electrochemical performances of the ZNW–Ni and ZP electrodes were also tested. Fig. 7c and e show the voltage versus capacity profiles of ZNW–Ni and ZP at 0.1 A g−1, respectively. The initial discharge and charge capacities for the ZNW–Ni electrode were 1814 and 1287 mA h g−1 respectively, and 1515 and 1110 mA h g−1 for the ZP electrode. For the ZNW–Ni electrode at 0.5 A g−1, at the beginning, its reversible discharge capacity of 1094 mA h g−1 was just a little lower than that of the ZNWG–Ni electrode. However, from the 20th cycle on, the capacity began to fall, and after only 50 cycles, it dropped to 742 mA h g−1, merely retaining 67.8% of its initial capacity, as shown in Fig. 7d. In a much worse manner, for the ZP electrode as shown in Fig. 7f, its initial reversible discharge capacity was only 745 mA h g−1, and what’s more, the capacity began to drop from the 20th cycle, with only 405 mA h g−1 (54.4% of the initial reversible capacity) retained after 50 cycles. These comparison measurements demonstrated that the performance of the ZNWG–Ni electrode is much higher than both the ZNW–Ni electrode and the pasted electrode of ZP.
Further demonstration of the remarkable performance of the ZNWG–Ni electrode was conducted by studying the electrochemical property of rate capability from a current density of 0.1 A g−1 to 1 A g−1, as shown in Fig. 7h. With the current density varying, following a 0.1 to 0.2 to 0.5 to 1 to 2 A g−1 increase, the corresponding discharge capacity followed a decrease of 1396 to 1288 to 1153 to 932 to 640 mA h g−1, respectively. Even at a high rate of 2 A g−1, it could still provide a reversible capacity of 640 mA h g−1, much higher than that of 372 mA h g−1 for graphite. Moreover, the discharge capacity could be restored to 1171 mA h g−1 and maintained when the current density changed back to 0.2 A g−1 after the high rate cycles above. However, for the ZNW–Ni electrode, when the current density increased following the same variation above, the capacity decreased from 1303 to 1201 to 1021 to 803 to 481 mA h g−1, with only 826 mA h g−1 left when the current density changed back to 0.2 A g−1. Things were worse for the ZP electrode, with a decrease from 1085 to 941 to 774 to 553 to 253 mA h g−1 following the same variation, with only 582 mA h g−1 remaining at 0.2 A g−1 again, which was inferior to the ZNWG–Ni electrode. Additionally, even comparing to other works reported previously, our ZNWG electrode can still keep ahead (Table S2†).
The high capacity of the ZNWG–Ni electrode could be due to its 3D porous nanowire structure and the direct growth on the Ni-foam substrate. The pores in the nanowire structure notably increased the material–electrolyte contact area and gave access to more Li-ion discharge/charge sites,48,53–55 meanwhile the direct growth could made a better contact to the current collector, thus shortening the charge transfer pathway and lowering the Li+ exchange resistance between the active material and electrolyte.4,54,55 These both achieved efficient electron conduction and collection to the current collector, and accommodated the stress arising from the volume change during the Li+ intercalation and de-intercalation process with more void space. It is worth mentioning that the capacity of rGO is only 33 mA h g−1 at the rate of 0.5 A g−1, as shown in Fig. S3.† Together with the similar capacities of the ZNW–Ni and ZNWG–Ni electrodes, we could say that rGO contributed little to the capacities. Moreover, rGO played a big role in improving the cyclability and rate capability, as the comparison in Fig. 7 shows. The superior cycling stability and fine rate capability were not only on account of the large material–electrolyte contact area and full contact with the conducting substrate, but were also ascribed to the presence of rGO covering the surface of the ZnCo2O4 nanowires. It could be inferred that rGO prevented the ZnCo2O4 nanowires from collapsing and falling out of the Ni-foam by wrapping the nanowires and covering them tightly to the substrate, as Fig. 3 shows, yet for the non-rGO covered electrodes, for instance both ZNW–Ni and ZP, the structure would fall down and peel off from the substrate after several cycles, leading to quick capacity fading. In order to prove this inference, SEM measurements were carried out again to obtain images of both ZNW and ZNWG on Ni-foam after 50 cycles at a current density of 0.5 A g−1, as shown in Fig. S4.† It is obvious that the shape of ZNW changed and the structure evidently collapsed after 50 cycles, while that of ZNWG shows little change, with only a bit of fracture in the nanowires.
Further characterization with electrochemical impedance spectroscopy (EIS) of the ZNWG–Ni, ZNW–Ni and ZP electrodes was carried out. The Nyquist plots of the three electrodes after the first cycle are shown in Fig. 8, which display a semicircle and a straight line in the high and low frequency regions, respectively. The relevant solution resistance (Rs) and calculated charge transfer resistance (Rct) of the cells are shown in Table S3.† It is obvious that the charge transfer resistance of the ZNWG electrode initially and after 100 cycles is smaller than the corresponding ones for both the ZNW and ZP electrodes. In the meantime, the increase in Rct after long cycling is also smaller than that of both the ZNW and ZP electrodes. Therefore, the ZNWG electrode can maintain its integrity better, leading to a longer cycle life.
Conclusions
In summary, we prepared a hierarchical ZnCo2O4 nanowire structure covered by rGO directly on a Ni-foam substrate for lithium ion batteries, with no binder or conductive agent, through a two-step facile fabrication using a hydrothermal method together with calcination, followed by electrochemical deposition. Compared with bare ZnCo2O4 nanowires grown on Ni-foam and ZnCo2O4 powder with binder and conductive additive, and even many reported works, it displayed a high specific capacity, excellent cycling stability and fine rate capability. A high capacity of 1170 mA h g−1 was reached and this remained at 1032 mA h g−1 even after 100 cycles at 0.3 A g−1. After a series of high rate tests and being returned to a low rate of 0.2 A g−1, it could still maintain a reversible capacity of 1171 mA h g−1. The good electrochemical performance of our electrode could be on account of not only the full material–electrolyte contact and available areas for Li+ intercalation and de-intercalation, the accommodation of the stress arising from the volume change and the efficient electron conduction and collection to the current collector provided by the hierarchical and porous structure, but also the presence of rGO that prevented the nanowires from collapsing and falling out from the Ni-foam, by wrapping the nanowires and covering them tightly to the substrate. This will be a promising anode material for high performance lithium ion batteries.Acknowledgements
The authors acknowledge the financial support from the National Natural Science Foundation of China (No. 21275104 and 21506131) and Science and Technology Support Program of Sichuan Province (No. 2015RZ0057).Notes and references
- K. Kang, Y. S. Meng, J. Bréger, C. P. Grey and G. Ceder, Science, 2006, 311, 977–980 CrossRef CAS PubMed.
- B. Scrosati, Nat. Nanotechnol., 2007, 2, 598–599 CrossRef CAS PubMed.
- M. Armand and J.-M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- K. T. Nam, D. W. Kim, P. J. Yoo, C. Y. Chiang, N. Meethong, P. T. Hammond, Y. M. Chiang and A. M. Belcher, Science, 2006, 312, 885–888 CrossRef CAS PubMed.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. Tarascon, Nature, 2000, 407, 496–499 CrossRef CAS PubMed.
- A. S. Arico, P. Bruce, B. Scrosati, J. M. Tarascon and W. Van Schalkwijk, Nat. Mater., 2005, 4, 366–377 CrossRef CAS PubMed.
- B. Kang and G. Ceder, Nature, 2009, 458, 190–193 CrossRef CAS PubMed.
- D. V. Bavykin, J. M. Friedrich and F. C. Walsh, Adv. Mater., 2006, 18, 2807–2824 CrossRef CAS.
- P. L. Taberna, S. Mitra, P. Poizot, P. Simon and J. M. Tarascon, Nat. Mater., 2006, 5, 567–573 CrossRef CAS PubMed.
- N. Kang, J. H. Park, J. Choi, J. Jin, J. Chun, I. G. Jung, J. Jeong, J. G. Park, S. M. Lee and H. J. Kim, Angew. Chem., 2012, 124, 6730–6734 CrossRef.
- G. R. Patzke, Y. Zhou, R. Kontic and F. Conrad, Angew. Chem., Int. Ed., 2011, 50, 826–859 CrossRef CAS PubMed.
- R. Alcántara, M. Jaraba, P. Lavela and J. Tirado, Chem. Mater., 2002, 14, 2847–2848 CrossRef.
- B. Liu, J. Zhang, X. Wang, G. Chen, D. Chen, C. Zhou and G. Shen, Nano Lett., 2012, 12, 3005–3011 CrossRef CAS PubMed.
- L. Hu, B. Qu, C. Li, Y. Chen, L. Mei, D. Lei, L. Chen, Q. Li and T. Wang, J. Mater. Chem. A, 2013, 1, 5596–5602 CAS.
- P. Meduri, E. Clark, J. H. Kim, E. Dayalan, G. U. Sumanasekera and M. K. Sunkara, Nano Lett., 2012, 12, 1784–1788 CrossRef CAS PubMed.
- S. Xiong, J. S. Chen, X. W. Lou and H. C. Zeng, Adv. Funct. Mater., 2012, 22, 861–871 CrossRef CAS.
- Y. Sharma, N. Sharma, G. Subba Rao and B. Chowdari, Adv. Funct. Mater., 2007, 17, 2855–2861 CrossRef CAS.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. Morozov, D. Jiang, Y. Zhang, S. A. Dubonos, I. Grigorieva and A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- F. Schedin, A. Geim, S. Morozov, E. Hill, P. Blake, M. Katsnelson and K. Novoselov, Nat. Mater., 2007, 6, 652–655 CrossRef CAS PubMed.
- H. A. Becerril, J. Mao, Z. Liu, R. M. Stoltenberg, Z. Bao and Y. Chen, ACS Nano, 2008, 2, 463–470 CrossRef CAS PubMed.
- C. Gómez-Navarro, R. T. Weitz, A. M. Bittner, M. Scolari, A. Mews, M. Burghard and K. Kern, Nano Lett., 2007, 7, 3499–3503 CrossRef PubMed.
- L. Chen, Y. Tang, K. Wang, C. Liu and S. Luo, Electrochem. Commun., 2011, 13, 133–137 CrossRef CAS.
- M. Hilder, B. Winther-Jensen, D. Li, M. Forsyth and D. R. MacFarlane, Phys. Chem. Chem. Phys., 2011, 13, 9187–9193 RSC.
- C. Liu, K. Wang, S. Luo, Y. Tang and L. Chen, Small, 2011, 7, 1203–1206 CrossRef CAS PubMed.
- Y. Yu, L. Gu, C. Zhu, S. Tsukimoto, P. A. van Aken and J. Maier, Adv. Mater., 2010, 22, 2247–2250 CrossRef CAS PubMed.
- W. Zhou, C. Cheng, J. Liu, Y. Y. Tay, J. Jiang, X. Jia, J. Zhang, H. Gong, H. H. Hng and T. Yu, Adv. Funct. Mater., 2011, 21, 2439–2445 CrossRef CAS.
- A. Pan, H. B. Wu, L. Yu, T. Zhu and X. W. Lou, ACS Appl. Mater. Interfaces, 2012, 4, 3874–3879 CAS.
- L. Li, Y. Cheah, Y. Ko, P. Teh, G. Wee, C. Wong, S. Peng and M. Srinivasan, J. Mater. Chem. A, 2013, 1, 10935–10941 CAS.
- X. Li, A. Dhanabalan, L. Gu and C. Wang, Adv. Energy Mater., 2012, 2, 238–244 CrossRef CAS.
- J. Jiang, Y. Li, J. Liu and X. Huang, Nanoscale, 2011, 3, 45–58 RSC.
- W. S. Hummers Jr and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef.
- N. I. Kovtyukhova, P. J. Ollivier, B. R. Martin, T. E. Mallouk, S. A. Chizhik, E. V. Buzaneva and A. D. Gorchinskiy, Chem. Mater., 1999, 11, 771–778 CrossRef CAS.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS PubMed.
- K. N. Kudin, B. Ozbas, H. C. Schniepp, R. K. Prud’Homme, I. A. Aksay and R. Car, Nano Lett., 2008, 8, 36–41 CrossRef CAS PubMed.
- D. V. Kosynkin, A. L. Higginbotham, A. Sinitskii, J. R. Lomeda, A. Dimiev, B. K. Price and J. M. Tour, Nature, 2009, 458, 872–876 CrossRef CAS PubMed.
- S. Vijayanand, P. A. Joy, H. S. Potdar, D. Patil and P. Patil, Sens. Actuators, B, 2011, 152, 121–129 CrossRef CAS.
- I. Grohmann, B. Peplinski and W. Unger, Surf. Interface Anal., 1992, 19, 591–594 CrossRef CAS.
- B. Varghese, C. Teo, Y. Zhu, M. V. Reddy, B. V. Chowdari, A. T. S. Wee, V. Tan, C. T. Lim and C. H. Sow, Adv. Funct. Mater., 2007, 17, 1932–1939 CrossRef CAS.
- U. Zielke, K. Hüttinger and W. Hoffman, Carbon, 1996, 34, 983–998 CrossRef CAS.
- C. Yuan, L. Yang, L. Hou, J. Li, Y. Sun, X. Zhang, L. Shen, X. Lu, S. Xiong and X. W. D. Lou, Adv. Funct. Mater., 2012, 22, 2560–2566 CrossRef CAS.
- B. Li, H. Cao, J. Shao, G. Li, M. Qu and G. Yin, Inorg. Chem., 2011, 50, 1628–1632 CrossRef CAS PubMed.
- C. Kim, M. Noh, M. Choi, J. Cho and B. Park, Chem. Mater., 2005, 17, 3297–3301 CrossRef CAS.
- W. Luo, X. Hu, Y. Sun and Y. Huang, J. Mater. Chem., 2012, 22, 8916–8921 RSC.
- N. Du, Y. Xu, H. Zhang, J. Yu, C. Zhai and D. Yang, Inorg. Chem., 2011, 50, 3320–3324 CrossRef CAS PubMed.
- D. Deng and J. Y. Lee, Nanotechnology, 2011, 22, 355401 CrossRef PubMed.
- J. Liu, J. Jiang, C. Cheng, H. Li, J. Zhang, H. Gong and H. J. Fan, Adv. Mater., 2011, 23, 2076–2081 CrossRef CAS PubMed.
- B. Liu, X. Wang, B. Liu, Q. Wang, D. Tan, W. Song, X. Hou, D. Chen and G. Shen, Nano Res., 2013, 6, 525 CrossRef CAS.
- P. Balaya, H. Li, L. Kienle and J. Maier, Adv. Funct. Mater., 2003, 13, 621–625 CrossRef CAS.
- X. Wang, X. Li, X. Sun, F. Li, Q. Liu, Q. Wang and D. He, J. Mater. Chem., 2011, 21, 3571–3573 RSC.
- G. Zhou, D. W. Wang, F. Li, L. Zhang, N. Li, Z. S. Wu, L. Wen, G. Q. Lu and H. M. Cheng, Chem. Mater., 2010, 22, 5306–5313 CrossRef CAS.
- Y. Wang, H. J. Zhang, L. Lu, L. P. Stubbs, C. C. Wong and J. Lin, ACS Nano, 2010, 4, 4753–4761 CrossRef CAS PubMed.
- G. Q. Zhang, H. B. Wu, H. E. Hoster, M. B. Chan-Park and X. W. D. Lou, Energy Environ. Sci., 2012, 5, 9453–9456 CAS.
- Y. G. Wang, H. Q. Li and Y. Y. Xia, Adv. Mater., 2006, 18, 2619–2623 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra21916g |
| This journal is © The Royal Society of Chemistry 2016 |