Abstract
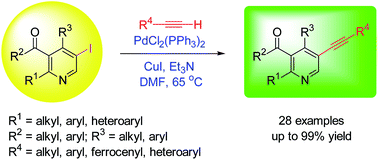
A facile and efficient synthetic route to densely substituted alkynylpyridines via a Sonogashira approach is reported. When treated with terminal alkynes in the presence of 5 mol% PdCl2(PPh3)2, 5 mol% CuI and excess Et3N in DMF at 65 °C, iodopyridines underwent Sonogashira coupling to afford alkynyl-substituted pyridines in good to excellent yields. The coupling reaction has been found to be general for a wide range of iodopyridines and terminal alkynes, and tolerated the presence of aliphatic, aromatic, heteroaromatic and ferrocenyl groups with electron-withdrawing and electron-donating substituents. This coupling approach could allow for the rapid construction of a library of functionalized alkynylpyridines of pharmacological interest.

 Please wait while we load your content...
Please wait while we load your content...