DOI:
10.1039/C5RA21651F
(Paper)
RSC Adv., 2016,
6, 19878-19886
Fabrication and excellent visible-light-driven photodegradation activity for antibiotics of SrTiO3 nanocube coated CdS microsphere heterojunctions
Received
17th October 2015
, Accepted 24th January 2016
First published on 1st February 2016
Abstract
Extending the light absorption range and facilitating the separation of photoinduced carriers are effective strategies in the process of improving photocatalysis. An effective method is to construct a heterostructure. In this work, a new heterojunction, CdS/SrTiO3, was synthesized via a two step hydrothermal route and designed to decontaminate hazardous wastewater containing antibiotics under visible light irradiation. Specially, the efficient photocatalytic performance of the composites could be ascribed to the enhancement of visible light absorption efficiency and the efficient separation of the photoexcited carriers originating from the heterointerface. Furthermore, a plausible photocatalytic mechanism has been discussed in detail based on trap experiments and ESR analysis results. The CdS/SrTiO3 heterojunction has potential to be applied for purifying antibiotic pollutants in environmental wastewater because of its high efficiency and stability.
1. Introduction
At present, the mishandling of pharmaceuticals, especially antibiotics, in wastewaters has caused a serious environmental problem worldwide owing to their high consumption. When these antibiotics are ingested by humans and livestock, they are partially excreted from the body as either metabolites or unmodified parent compounds into environmental waters in their pharmaceutically active form, which may be a case of alarm for aquatic ecosystems as well as public health.1–4 Therefore, the development of efficient treatments for eliminating antibiotics left in the ecological environment is an urgent task. Semiconductor photocatalysis has been considered an effective process in the mitigation of environmental issues resulting from the occurrence of organic contaminants in the environment using solar energy.5–8 The unremitting efforts over the past forty years have made significant achievements in the semiconductor photocatalytic degradation fields, just as the literature has reported.9–11 However, most of the reports for applications in degrading organic pollutants mainly focused on degradation of a single contaminant. Hazardous wastewater usually contains multiple antibiotic pollutants. Therefore, exploiting new photocatalysts not confined to a single contaminant is of high interest and has an important significance.
To date, the semiconductor SrTiO3 has undoubtedly been proven to be a renowned photocatalyst due to its excellent chemical stability, non-toxicity, low cost and favorable optoelectronic properties, and is deemed to be a promising photocatalyst for water splitting, inactivating viruses and completely eliminating organic contaminants.12–16 However, as a model ultraviolet (UV) photocatalyst similar to TiO2, SrTiO3 is particularly suitable for applications based on UV-light irradiation, which occupies only 4% of the incoming solar light spectrum on earth. In order to push the absorption onset of SrTiO3 toward longer wavelengths that cover the largest proportion of the solar spectrum, strenuous efforts have been made, including doping,17,18 suitable textural design,19,20 and coupling with metal21 or other semiconductors.22,23 Among these, designing composite materials with matched band-edges is an important and effective strategy because it facilitates the immigration and separation of photo-generated electrons and holes, leading to an improved overall efficiency.24–26 Some composites of SrTiO3 (SrTiO3/Fe3O4,27 SrTiO3/Cu2O,28 Ag3PO4/SrTiO3 (ref. 29)) have also been reported for the enhancement of photocatalytic O2 and H2 production activity or organic pollutant elimination ability under visible light irradiation. Therefore, fabricating a new composite heterostructure is a tempting method to sensitize SrTiO3 with higher visible light absorption and electron migrating efficiency. CdS has been extensively researched as a common photocatalyst for its favorable band gap (2.42 eV), whose conduction band (CB) and valence band (VB) are both negative compared to those of SrTiO3.30–32 Thus, it is conjectured that the heterostructure constructed between CdS and SrTiO3 will not only extend the absorption of SrTiO3 to the visible light region but may also boost electron transport from the CB of CdS to that of SrTiO3. Such a result would significantly enhance the photocatalytic activity. To our knowledge, no prior works concerning the employment of a CdS/SrTiO3 heterojunction as an effective photocatalyst for degrading antibiotics have been reported to date.
Inspired by the above, we herein fabricated a CdS/SrTiO3 heterojunction via a surface modification strategy using a two-step simple hydrothermal method for the first time. It demonstrated superior photocatalytic activity in degrading five antibiotics under visible light irradiation; ciprofloxacin (CIP), enrofloxacin hydrochloride (ENR), oxytetracycline (OTC), danofloxacin mesylate (DAN) and levofloxacin (LEV). Specially, the effect of the CdS microsphere content on the photocatalytic activity was evaluated and the appropriate content of CdS microspheres (60 wt%) plays an essential role in improving the synergetic effects on the transfer of the carriers. In addition, the stability of the CdS/SrTiO3 heterojunction photocatalysts was monitored by circulation experiments. This work demonstrates that CdS/SrTiO3 heterostructures are highly efficient, stable, and reusable photocatalysts for potential application in wastewater treatment.
2. Experimental
2.1. Materials
Titania TiO2 (P25) powder was purchased from Degussa (Germany). Sr(OH)2·8H2O, KOH, Cd(CH3COO)2·2H2O and thiourea (CN2H4S) were purchased from Aladdin (Shanghai, China). All reagents were analytically grade and used without further purification.
2.2. Preparation of the photocatalyst
Pure phase SrTiO3, with a particle size of approximately 50 nm, was synthesized by a hydrothermal method. In a typical synthesis, 3 mmol of Sr(OH)2 was dissolved in 35 mL of distilled water keeping vigorous stirring to form a suspension solution, to which 3.5 mmol of P25 was added, and 2 g of KOH was finally added into the mixture keeping pH 13.0. After stirring for 30 min, the mixture was transferred into a 50 mL Teflon-lined stainless autoclave for hydrothermal treatment at 150 °C for 72 h. A white precipitate was obtained by centrifugation and then washed with distilled water and ethanol until the pH reached 7 and afterward, it was dried overnight at 60 °C. The SrTiO3/CdS heterostructures were synthesized by a hydrothermal method too. Using SrTiO3/CdS-60 wt% as a model: 0.1835 g of the as-prepared SrTiO3 nanocubes was dispersed into 50 mL of distilled water and ultrasonically treated for 10 min. Then 0.505 g of Cd(CH3COO)2·2H2O and 0.288 g CN2H4S (Cd2+/S2− = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were mixed and dissolved by stirring for 1 h. Subsequently, the mixture was loaded in a 100 mL Teflon-lined stainless steel autoclave. The autoclave was sealed and heated in a program-controlled oven at 180 °C for 10 h and then air-cooled to room temperature. The resultant products were separated by centrifugation, washed with distilled water and ethanol, and dried in a vacuum at 60 °C for one night. We also conducted a hydrothermal method for the synthesis of pure CdS microspheres using the same method as above in the absence of SrTiO3, which is reported in the literature.32
2) were mixed and dissolved by stirring for 1 h. Subsequently, the mixture was loaded in a 100 mL Teflon-lined stainless steel autoclave. The autoclave was sealed and heated in a program-controlled oven at 180 °C for 10 h and then air-cooled to room temperature. The resultant products were separated by centrifugation, washed with distilled water and ethanol, and dried in a vacuum at 60 °C for one night. We also conducted a hydrothermal method for the synthesis of pure CdS microspheres using the same method as above in the absence of SrTiO3, which is reported in the literature.32
The CdS/SrTiO3 heterojunctions were obtained by simply adjusting the usage of CdS and were labeled as SC-x, in which x refers to the CdS loading amount (10 wt%, 40 wt%, 60 wt%, 70 wt% and 80 wt%).
2.3. Photocatalyst characterization
Powder X-ray diffraction (XRD) patterns were obtained with a D/MAX-2500 diffractometer (Rigaku, Japan) with Cu Kα radiation from 10° to 80° with a step size of 0.02° at a scan rate of 5° min−1. Transmission electron microscopy (TEM), high-resolution TEM (HR-TEM) and high-angle annular dark-field scanning TEM (HAADF-STEM) were performed on JEM-2100 (HR) microscopes with a field-emission gun and the acceleration voltage for both microscopes was 200 kV. Scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS), were used to characterization the samples. UV-vis absorption spectra of the samples were recorded with a Shimadzu UV-2500 spectrophotometer using BaSO4 as reference. X-ray photoelectron spectroscopy (XPS) data of the samples were obtained by a Thermo ESCALAB 250X (America) electron spectrometer using 150 W Al Kα radiation. Electron spin resonance (ESR) analysis was performed using a Bruker EPR A300-10/12 spectrometer.
2.4. Photocatalytic activity studies
The photocatalytic activities of the as-prepared photocatalysts were estimated by degrading multiple antibiotics using a photochemical reactor under visible light irradiation (250 W Xe lamp with a cut-off filter at 400 nm). Under such conditions, the optical power density was 150 mW cm−2 (CEL-NP2000). Firstly, using CIP as an example, for each experiment, 100 mg of photocatalyst was added into a CIP aqueous solution (10 mg L−1, 100 mL). Prior to irradiation, the suspensions were magnetically stirred in the dark for 30 min to achieve absorption–desorption equilibrium between the photocatalysts and pollutants. During the degradation progress, 5 mL of the suspension was sampled in 20 min intervals and centrifuged (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 5 min) to remove the photocatalyst particles. The concentration of aqueous CIP was determined with a spectrometer by measuring the absorbance at 277 nm.33 The photocatalytic degradation ratio (DR) was calculated by the following formula:
000 rpm, 5 min) to remove the photocatalyst particles. The concentration of aqueous CIP was determined with a spectrometer by measuring the absorbance at 277 nm.33 The photocatalytic degradation ratio (DR) was calculated by the following formula:
| DR% = 100% − (Ai/A0) × 100% |
where A0 is the initial absorbance of CIP at absorption equilibrium, and Ai is the absorbance of the degraded solution at a certain number of minutes.
Additionally, in order to verify the universality of the as-prepared CdS/SrTiO3 heterojunction, the degradation of four other antibiotics, ENR, OTC, DAN and LEV were further estimated under visible light. The operational processes were the same as the CIP degradation. The concentrations of ENR, OTC, DAN and LEV were also monitored using a UV-vis spectrophotometer at the wavelengths of 275 nm,34 355 nm,35 282 nm and 294 nm, respectively.
2.5. Photocurrent measurements
Photocurrent density measurements were carried out in a prescriptive three-electrode system employing a Pt wire as the counter electrode, the samples as the working electrode and Ag/AgCl (saturated KCl) as the reference electrode. Transparent conductive fluorine-doped tin oxide (FTO) coated glass of size 10 mm × 30 mm × 2 mm was used as the substrate for the deposition of the films. Before deposition of the photocatalysts onto FTO coated glass, the substrate was cleaned in an ultrasonic bath using acetone, chloroform, ethanol and distilled water each for 30 min. The as-prepared photocatalyst film electrodes on FTO coated glass served as the working electrode. The working electrode was prepared by a simple casting method as follows: 0.3 g of each sample and 0.02 g of polyethylene glycol were dispersed into 3 mL of ethanol and 0.03 mL of oleic acid to form a slurry.36 Then the slurry was dropped onto the FTO coated glass via a drop-casting method. The electrodes were then dried in a tube furnace, and calcined at 350 °C for 2 h under a flow of N2 gas. The photocurrent was collected and measured via electron shuttles on an inert Pt electrode immersed in an electrolytic tank with 0.5 M Na2SO4 aqueous solution at a 0.5 V potential bias under a 300 W xenon lamp exposure.
3. Results and discussion
3.1. Structural characterization of the as-prepared photocatalysts
X-ray diffraction (XRD) was used to investigate the phase composition, purity, and crystallinity of the resulting products. Fig. 1a shows the XRD patterns of CdS, CdS/SrTiO3, and SrTiO3. Six diffraction peaks appear at 2θ = 22.8°, 32.4°, 40°, 46.5°, 57.8°, 67.8° and 77.2°, which coincide with the diffraction patterns of SrTiO3 (ICDD PDF#84-0444). The major diffraction peaks at 2θ values of 24.8°, 26.5°, 28.2°, 43.7° and 52.9° are indexed to the (100), (002), (101), (110) and (201) crystallographic planes of CdS (ICDD PDF#75-2306), respectively. The XRD pattern of the CdS/SrTiO3 composite is similar to that for SrTiO3 and CdS, which shows a combination of SrTiO3 and CdS, and rules out the possibility of other impurity phases, indicating successful construction of the heterojunction. The elemental analysis of the CdS/SrTiO3 heterojunction was carried out by EDS, as shown in Fig. 1b, from which the elements Cd, S, Sr, O and Ti can be directly observed.
 |
| | Fig. 1 (a) XRD patterns of SrTiO3 (STO), CdS and CdS/SrTiO3; (b) EDS spectrum of SC-60. | |
To investigate the surface morphology and particle size of the representative photocatalysts, typical electron microscope images were obtained. From the SEM image in Fig. 2a, it is clear that pure CdS possesses uniform-sized microspheres of approximately 300 nm in diameter. The TEM image in Fig. 2b shows that the pure SrTiO3 particles display highly uniform and regular cubes with diameters in the range of 20–50 nm. To provide further investigation into the interface contact features of the as-prepared heterojunction, TEM and HR-TEM were carried out. From Fig. 2c, it can be seen that the SrTiO3 nanocubes grow on the CdS microspheres. The TEM image in Fig. 2d further demonstrates the close connection between the SrTiO3 nanocubes and CdS microspheres, which is beneficial to interparticle photo-induced carrier transfer. The detailed nano-junction structure is displayed via a typical HR-TEM image in Fig. 2d. The d-spacings of 0.299 and 0.289 nm match the (101) lattice plane of CdS and the (110) lattice plane of SrTiO3, respectively. Fig. 3 displays the HAADF-STEM imaging of the CdS/SrTiO3 heterojunction to identify the composition and element distribution. Elements corresponding to O, Sr, Ti, Cd and S are observed, which are derived from SrTiO3 and CdS, respectively. Further, the results of the HAADF-STEM elemental maps also confirm the SrTiO3 nanocubes are uniformly distributed on the surface of the CdS microspheres.
 |
| | Fig. 2 (a) SEM images of pure CdS; TEM images of (b) pure SrTiO3, (c) SC-60 and (d) HRTEM images of SC-60. | |
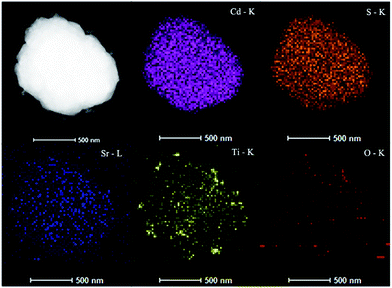 |
| | Fig. 3 HADDF-STEM image of the SC-60 sample with maps of Cd–K, S–K, Sr–L, Ti–K and O–K. | |
XPS spectra were recorded to provide the surface compositions and chemical states present in the CdS/SrTiO3 heterojunction. The full survey spectrum (Fig. 4a) shows that the sample is composed of the elements Cd, S, Sr, Ti and O, and no other impurities were detected, which agrees well with the results of EDS. Fig. 4b shows the high-resolution Cd 3d XPS spectrum of the CdS/SrTiO3 heterojunction, the peaks at 405.9 eV and 412.9 eV are ascribed to Cd 3d3/2 according to the standard database, which can be attributed to Cd2+ in CdS. From Fig. 4c, it can be seen that the peak of the binding energy for S 2p3/2 is located at 161.5 eV, which is assigned to S2− ions. Two strong peaks centered at 133.2 eV and 134.9 eV are observed in Fig. 4d, belonging to the Sr 3d5/2 and Sr 3d3/2 states, respectively, which implies that the main elemental chemical states are divalent and bonds with titanates.37 From the XPS spectrum of Ti 2p shown in Fig. 4e, the bonding energy of the Ti 2p3/2 and Ti 2p1/2 peaks at 458.4 eV and 463.6 eV are assigned to Ti4+, which results from SrTiO3. The XPS spectrum of O 1s was found at the binding energy of 529.2 eV. All above results show that the CdS/SrTiO3 heterojunction was successfully prepared by the two step hydrothermal route.
 |
| | Fig. 4 XPS spectra of SC-60: (a) survey spectrum, (b) Cd 3d, (c) S 2p, (d) Sr 3d, (e) Ti 2p and (f) O 1s. | |
3.2. UV-vis adsorption of as-prepared photocatalysts
The UV-vis absorption spectra of the as-synthesized samples in pressed disk form were recorded on a Shimadzu UV-2500 spectrophotometer equipped with an integrating sphere assembly, over a wavelength range of 200–700 nm employing BaSO4 as a reflectance standard. The pressed disk was composed of BaSO4 powders and the as-synthesized samples: BaSO4 powders were flattened on a diffusion reflective cell, 50 mg of the as-synthesized samples were then pressed on the center of the diffusion reflective cell. The light absorption properties of the prepared SrTiO3, CdS and CdS/SrTiO3 heterojunction are shown in Fig. 5. The spectrum of pure SrTiO3 has a sharp absorption edge at ∼380 nm, corresponding to the band gap energy of 3.2 eV. The absorption spectrum of CdS indicates that it can absorb visible light with an absorption edge at ∼580 nm. Therefore, in contrast to CdS and SrTiO3, the absorption characteristic of the CdS/SrTiO3 heterojunction is a little different, which can be attributed to the interfacial interaction between SrTiO3 and CdS and the photosensitizing effect of the incorporated CdS microspheres.38
 |
| | Fig. 5 UV-vis absorption spectra of SrTiO3, CdS and SC-60. | |
3.3. Photocatalytic activity and the stability of the as-prepared photocatalysts
To elucidate the photocatalytic activities of the as-prepared samples for antibiotic degradation, we performed an investigation into removing CIP as a model pollutant under visible light irradiation (λ > 420 nm) for 120 min, and the results are shown in Fig. 6a. There are 7 degradation curves in Fig. 6a, the vertical coordinates represent the degradation ratios, simply “DRs” for short. The CIP degradation rate of the bare SrTiO3 nanocubes is inappreciable because it can only be excited by UV light. In addition, the pure CdS microspheres reveal feeble photocatalytic performance for removing CIP (degradation rate of ∼44.3%), which may be attributed to the high recombination rate of the photo-generated electrons and holes. However, the performance of the CdS/SrTiO3 heterojunction, by contrast, exhibits a remarkable enhancement for degrading CIP because of the enhanced charge separation efficiency resulting from its heterojunction structure. This is based on the fact that CdS-sensitized semiconductor composites can shuttle the photoexcited electrons from the CB of CdS to the CB of SrTiO3,38 which facilitates separation of the electrons and holes, thus improving the degradation of CIP. Interestingly, Fig. 6b shows the activity of the CdS/SrTiO3 heterojunction for degrading CIP is enhanced rapidly with increasing content of CdS and then gradually decreases with further loading. When the content of CdS increases up to 60 wt% (SC-60), the degradation rate of CIP reaches to ∼93.7%. This demonstrates that the CdS loading amount strongly affects the photocatalytic activity.
 |
| | Fig. 6 Photocatalytic activity towards the degradation of CIP with as-prepared samples under visible light irradiation. | |
Considering the complexity of practical wastewater, another four typical antibiotics, ENR, OTC, DFM and LEV, were selected as target pollutants for further evaluating the photocatalytic activity of the SC-60 heterojunction. As shown in Fig. 7, the SC-60 heterojunction exhibits high photocatalytic activity for ENR, OTC, DAN and LEV mineralization (91.1%, 90.3%, 91.5% and 88.6%) under visible light irradiation, which is similar to the degradation rate of CIP (93.7%). The results reveal that the CdS/SrTiO3 heterojunction displays excellent visible-light-driven photocatalytic activity and could be a promising candidate for application in environmental purification.
 |
| | Fig. 7 The photodegradation performance of the SC-60 sample for ENR, OTC, DAN and LEV degradation under visible light irradiation. | |
The reusability and stability of a photocatalyst are also of paramount importance for practical application. To evaluate the stability of the photocatalytic performance of the SC-60 heterojunction, five consecutive cycles of photocatalytic degradation of CIP were performed, using the same photocatalyst and a fresh CIP solution each time. The results of the cycling experiments are depicted in Fig. 8. The photocatalytic activity of the SC-60 heterojunction shows a small amount of deactivation in the photodegradation of CIP, which may originate from the unavoidable loss of the photocatalyst by centrifugation. Furthermore, the stability was analysed by observing the XRD of the photocatalyst before and after the photocatalytic recycles. As shown in Fig. 8b, the XRD diffraction patterns of the CdS/SrTiO3 heterojunction show almost no change after the photocatalytic cycles, implying that the CdS/SrTiO3 heterojunction is photostable and photocorrosion resistant.39 Hence, the CdS/SrTiO3 heterojunction may serve as a promising candidate for application in purification of antibiotic wastewater.
 |
| | Fig. 8 (a) Five reaction cycles for the photocatalytic degradation of CIP by SC-60, and (b) XRD patterns of SC-60 before and after photocatalytic reaction. | |
3.4. Photoelectrochemical properties of the as-prepared photocatalysts
The separation efficiency of the excited electrons and holes for the CdS/SrTiO3 heterojunction can be validated by photocurrent under 300 W xenon lamp irradiation. Considering the several on–off cycles of irradiation of transient photocurrent responses over the as-prepared electrodes, we utilized the photocurrent results to probe the fate of the photo-generated carriers.40 Higher photocurrent density causes enhanced transport and separation efficiency of the photo-generated carriers.41 As outlined in Fig. 9, the photocurrent density of the CdS/SrTiO3 heterojunction is significantly higher than that of bare CdS, meaning that it is more effective in separating the electron–hole pairs.42,43 In addition, the optimum loading amount of CdS microspheres of 60 wt% exhibits the highest photocurrent density, indicating the remarkable reduction in recombination of photogenerated electrons and holes, which is consistent with its superior photocatalytic activity.
 |
| | Fig. 9 Transient photocurrent response for the as-prepared samples. | |
3.5. Photocatalytic mechanism
To explore the mechanism of photocatalytic degradation of CIP utilizing the SC-60 heterojunction photocatalyst, a series of quenchers were deployed to determine the dominant oxidative species in the photocatalytic process, namely, adding benzoquinone (BQ), isopropanol (IPA), AgNO3 and triethanolamine (TEA) as a scavenger for ˙O2−,44,45 ˙OH,46 e− (ref. 47 and 48) and h+,49 respectively. Fig. 10 presents degradation kinetic curves of CIP over the SC-60 heterojunction under the different conditions. It is clear that when AgNO3 (e− scavenger) and BQ (˙O2− scavenger) are added, respectively, the photocatalytic degradations of CIP are significantly inhibited, which confirms the paramount role of e− and ˙O2− in the reaction process. Furthermore, the addition of IPA (˙OH scavenger) only leads to a small change in the photocatalytic degradation of CIP, indicating the contribution of ˙OH radicals is secondary. Further, the addition of TEA (h+ scavenger) which is used to remove h+ bound to the surface, only results in a tiny decrease in the photodegradation of CIP. Through this comparison, the importance of the activated species follows the order: ˙O2− > ˙OH > h+. In addition, based on the band gap positions, the CB and VB edge potentials of SrTiO3 were defined as −0.4 eV and 2.8 eV and those of CdS are −0.52 eV and 1.88 eV, respectively.38,50 The reduction potential of O2/˙O2− and ˙OH/H2O is −0.33 eV and 2.7 eV (versus NHE), respectively.51,52 Thus, under visible light irradiation, the electrons in the CB of CdS will transfer into that of SrTiO3 through the interface of the CdS/SrTiO3 heterojunction, which can afford to trap molecular oxygen to generate ˙O2− and transform into ˙OH. By contrast, ˙O2− and ˙OH cannot be generated from the CB of CdS directly. In addition, the holes play a small part in degrading CIP, which may result from the low potential of the VB of CdS and the weak oxidation ability of the generated holes. Eventually, we can conclude that the degradation of CIP over the SC-60 heterojunction is driven mainly by the participation of ˙O2−, secondarily by ˙OH, and to a lesser extent by the contribution of h+.
 |
| | Fig. 10 Photocatalytic degradation of CIP with different radical scavengers over SC-60 under visible light irradiation. | |
To further verify the generation of radicals (˙O2− and ˙OH), the ESR (electron spin resonance) of the SC-60 heterojunction was studied using dimethyl pyridine N-oxide (DMPO) as a trapping agent. Before the measurement, the samples (5 mg) and DMPO (30 μL) were dispersed in deionized water and methanol, respectively. The former was used to detect the hydroxyl radicals (DMPO-˙OH), and the latter was used to detect the superoxide radicals (DMPO-˙O2−).48,53 We conducted the same procedure for the pure CdS microspheres for comparison. As shown in Fig. 11, the ESR signals of ˙O2− and ˙OH were detected at the same conditions, and the signal intensity of DMPO-˙O2− was stronger than that of DMPO-˙OH for the SC-60 heterojunction, indicating that ˙O2− plays a leading role in the CIP degradation process, which is consistent with the previous trapping experiments. Further, the signal intensities of both DMPO-˙O2− and DMPO-˙OH of the pure CdS microspheres were weaker than the SC-60 heterojunction. Herein, we may safely draw the conclusion that the CdS/SrTiO3 heterojunction can be conducive to accelerating electron transport and separation.
 |
| | Fig. 11 DMPO spin-trapping ESR spectra of CdS and SC-60 for (a) DMPO-˙OH and (b) DMPO-˙O2− irradiated for 90 s. | |
Based on the above findings from the active species trapping experimental results and ESR analysis, a possible mechanism for the degradation of CIP over the SC-60 heterojunction is presented in Scheme 1. Under the visible light irradiation, CdS in SC-60 heterojunction can be excited to form electron–hole pairs. Simultaneously, photoexcited electrons in the CB of CdS can quickly transfer to the CB of SrTiO3. The electrons then diffuse to the surface of SrTiO3 to activate molecular oxygen to form ˙O2− and efficiently enhance the separation of charge carriers. A majority of ˙O2− are directly involved in the oxidation of CIP. A fraction of the formed ˙O2− contribute to the generation of ˙OH, which plays an assistant role in the photocatalytic degradation of CIP. The generated holes are released to the surface of the photocatalysts to participate in the degradation of CIP to some extent. The possible photocatalytic reaction process can be expressed as follows:
| CdS + hν → CdS (eCB− + hVB+) |
| CdS (eCB−) + SrTiO3 → CdS + SrTiO3 (eCB−) |
| SrTiO3 (eCB−) + O2 → SrTiO3 + ˙O2− |
| ˙O2− + 2H2O → 2˙OH + H2O2 |
| ˙O2− + CIP → degraded products |
| ˙OH + CIP → degraded products |
| CdS (h+) + CIP → degraded products |
 |
| | Scheme 1 Proposed reaction mechanisms for CIP degradation over SC-60 photocatalyst under visible light. | |
4. Conclusions
In this paper, a CdS/SrTiO3 heterojunction is obtained by surface modification of SrTiO3 nanocubes on CdS microspheres via a two step hydrothermal process. It shows improved photocatalytic activity for removing CIP pollutants under visible light irradiation, which is mainly ascribed to the heterostructure enhancing the separation efficiency of electrons and holes. Moreover, a possible photocatalytic mechanism is determined, confirming that ˙O2− radicals are the dominant active species to degrade CIP, and ˙OH radicals play an assistant role in the photocatalytic degradation process of CIP. It is worth noting that the CdS/SrTiO3 heterostructure is not just confined to the single degradation of CIP, but also exhibits universality for removing many kinds of antibiotics, such as ENR, OTC, DAN and LEV etc. This work provides an effective way to remove antibiotic pollutants in environmental wastewater owing to the high efficiency and stability of our obtained CdS/SrTiO3 heterojunction.
Acknowledgements
We gratefully acknowledge the financial support of the National Natural Science Foundation of China (21546006, 21276116, and 21477050), Excellent Youth Foundation of Jiangsu Scientific Committee (BK20140011), Chinese-German Cooperation Research Project (GZ1091), Henry Fok Education Foundation (141068), Six Talents Peak Project in Jiangsu Province (XCL-025), the Start-Up Foundation of Jiangsu University (11jdg104) and the Natural Science Foundation of Jiangsu Province (BK2012701).
References
- S. E. Jørgensen and B. Halling-Sørensen, Chemosphere, 2000, 40, 691 CrossRef
 .
. - A. Alighardashi, D. Pandolfi, O. Potier and M. N. Pons, J. Hazard. Mater., 2009, 72, 685 CrossRef PubMed
 .
. - S. D. Richardson and T. A. Ternes, Anal. Chem., 2011, 83, 4614 CrossRef CAS PubMed
 .
. - D. A. Keane, K. G. McGuigan, P. F. Ibáñez, M. I. Polo-López, J. A. Byrne, P. S. M. Dunlop, K. O’Shea, D. D. Dionysiou and S. C. Pillai, Catal. Sci. Technol., 2014, 4, 1211 CAS
 .
. - H. Wang, L. Zhang, Z. Chen, J. Hu, S. Li, Z. Wang, J. Liu and X. Wang, Chem. Soc. Rev., 2014, 43, 5234 RSC
 .
. - S. Y. Reece, J. A. Hamel, K. Sung, T. D. Jarvi, A. J. Esswein, J. J. H. Pijpers and D. G. Nocera, Science, 2011, 334, 645 CrossRef CAS PubMed
 .
. - H. Tong, S. Ouyang, Y. Bi, N. Umezawa, M. Oshikiri and J. Ye, Adv. Mater., 2012, 24, 229 CrossRef CAS PubMed
 .
. - M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemannt, Chem. Rev., 1995, 95, 69 CrossRef CAS
 .
. - J. Tiana, Y. H. Leng, Z. H. Zhao, Y. Xia, Y. H. Sang, P. Hao, J. Zhan, M. C. Li and H. Liu, Nano Energy, 2015, 11, 419–427 CrossRef
 .
. - G. G. Bessegato, J. C. Cardoso, B. F. Silva and M. V. B. Zanoni, Appl. Catal., B, 2016, 80, 161 CrossRef
 .
. - W. Y. Lu, T. F. Xu, Y. Wang, H. G. Hu, N. Li, X. M. Jiang and W. X. Chen, Appl. Catal., B, 2016, 80, 20 CrossRef
 .
. - S. Ouyang, H. Tong, N. Umezawa, J. Cao, P. Li, Y. Bi, Y. Zhang and J. Ye, J. Am. Chem. Soc., 2012, 134, 1974 CrossRef CAS PubMed
 .
. - T. K. Townsend, N. D. Browning and F. E. Osterloh, ACS Nano, 2012, 6, 7420–7426 CrossRef CAS PubMed
 .
. - S. Hara and H. Irie, Appl. Catal., B, 2012, 115, 330 CrossRef
 .
. - A. Iwase, Y. H. Ng, Y. Ishiguro, A. Kudo and R. Amal, J. Am. Chem. Soc., 2011, 133, 11054 CrossRef CAS PubMed
 .
. - U. Sulaeman, S. Yin and T. Sato, Appl. Catal., B, 2011, 102, 286 CrossRef CAS
 .
. - W. S. Choi, H. K. Yoo and H. Ohta, Adv. Funct. Mater., 2015, 5, 799 CrossRef
 .
. - R. B. Comes, P. V. Sushko, S. M. Heald, R. J. Colby, M. E. Bowden and S. A. Chambers, Chem. Mater., 2014, 26, 7073 CrossRef CAS
 .
. - S. Ouyang, P. Li, H. Xu, H. Tong, L. Liu and J. Ye, ACS Appl. Mater. Interfaces, 2014, 6, 22726 CAS
 .
. - Q. Kuang and S. Yang, ACS Appl. Mater. Interfaces, 2013, 5, 3683 CAS
 .
. - L. Liu, P. Li, B. Adisak, S. Ouyang, N. Umezawa, J. Ye, R. Kodiyath, T. Tanabe, G. V. Ramesh, S. Ueda and H. Abe, J. Mater. Chem. A, 2014, 2, 9875 CAS
 .
. - J. Guo, S. Ouyang, P. Li, Y. Zhang, T. Kako and J. Ye, Appl. Catal., B, 2013, 134, 286 CrossRef
 .
. - H. W. Kang, S. N. Lim, D. Song and S. B. Park, Int. J. Hydrogen Energy, 2012, 37, 11602 CrossRef CAS
 .
. - Y. Zhang, Q. Ji, G. Han, J. Jing, J. Shi, D. Ma, J. Sun, Y. Zhang, M. Li, X. Lang, Y. Zhang and Z. Liu, ACS Nano, 2014, 8, 8617 CrossRef CAS PubMed
 .
. - Q. Xiang, J. Yu and M. Jaroniec, J. Am. Chem. Soc., 2012, 134, 6575 CrossRef CAS PubMed
 .
. - T. K. Townsend, N. D. Browning and F. E. Osterloh, Energy Environ. Sci., 2012, 5, 9543 CAS
 .
. - A. M. Schultz, P. A. Salvador and G. S. Rohrer, Chem. Commun., 2012, 48, 2012 RSC
 .
. - D. Sharma, S. Upadhyay, V. R. Satsangi, R. Shrivastav, U. V. Waghmare and S. Dass, J. Phys. Chem. C, 2014, 118, 25320 CAS
 .
. - X. Guan and L. Guo, ACS Catal., 2014, 4, 3020 CrossRef CAS
 .
. - Z. Yu, F. Qu and X. Wu, Dalton Trans., 2014, 43, 4847 RSC
 .
. - S. C. Hayden, N. K. Allam and M. A. El-Sayed, J. Am. Chem. Soc., 2010, 132, 14406 CrossRef CAS PubMed
 .
. - Z. Yu, B. Yin, F. Qu and X. Wu, Chem. Eng. J., 2014, 258, 203 CrossRef CAS
 .
. - T. An, H. Yang, G. Li, W. Song, W. J. Cooper and X. Nie, Appl. Catal., B, 2010, 4, 288 CrossRef
 .
. - M. Sturini, A. Speltini, F. Maraschi, A. Profumo, L. Pretali, E. A. Irastorza, E. Fasani and A. Albini, Appl. Catal., B, 2012, 19, 32 CrossRef
 .
. - C. Zhao, M. Pelaez, X. Duan, H. Deng, K. O’Shea, D. Fatta-Kassinos and D. D. Dionysiou, Appl. Catal., B, 2013, 134, 83 CrossRef
 .
. - S. M. Liu, W. Y. Ding and W. P. Chai, Phys. B, 2011, 406, 2303 CrossRef CAS
 .
. - H. Yu, S. Ouyang, S. Yan, Z. Li, T. Yu and Z. Zou, J. Mater. Chem., 2011, 21, 11347 RSC
 .
. - S. Yu, Y. H. Kim, S. Y. Lee, H. D. Song and J. Yi, Angew. Chem., Int. Ed. Engl., 2014, 53, 11203 CrossRef CAS PubMed
 .
. - J. Song, Z. Luo, D. K. Britt, H. Furukawa, O. M. Yaghi, K. I. Hardcastle and C. L. Hill, J. Am. Chem. Soc., 2011, 133, 16839 CrossRef CAS PubMed
 .
. - M. Kong, Y. Z. Li, X. Chen, T. T. Tian, P. F. Fang, F. Zheng and X. J. Zhao, J. Am. Chem. Soc., 2011, 133, 16414 CrossRef CAS PubMed
 .
. - G. He, C. Xing, X. Xiao, R. Hu, X. Zuo and J. Nan, Appl. Catal., B, 2015, 70, 1 Search PubMed
 .
. - F. Dong, Z. Zhao, T. Xiong, Z. Ni, W. Zhang, Y. Sun and W. K. Ho, ACS Appl. Mater. Interfaces, 2013, 5, 11392 CAS
 .
. - H. Xu, J. Yan, Y. Xu, Y. Song, H. Li, J. Xia, C. Huang and H. Wan, Appl. Catal., B, 2013, 129, 182 CrossRef CAS
 .
. - J. Di, J. Xia, Y. Ge, H. Li, H. Ji, H. Xu, Q. Zhang, H. Li and M. Li, Appl. Catal., B, 2015, 168, 51 CrossRef
 .
. - S. Wang, D. Li, C. Sun, S. Yang, Y. Guan and H. He, Appl. Catal., B, 2014, 144, 885 CrossRef CAS
 .
. - G. Li, K. H. Wong, X. Zhang, C. Hu, J. C. Yu, R. C. Chan and P. K. Wong, Chemosphere, 2009, 76, 1185 CrossRef CAS PubMed
 .
. - Y. H. Zhang, N. Zhang, Z. R. Tang and Y. J. Xu, ACS Nano, 2012, 6, 9777 CrossRef CAS PubMed
 .
. - S. Liu, N. Zhang, Z. R. Tang and Y. J. Xu, ACS Appl. Mater. Interfaces, 2012, 4, 6378 CAS
 .
. - L. Q. Ye, J. Y. Liu, C. Q. Gong, L. H. Tian, T. Y. Peng and L. Zan, ACS Catal., 2012, 2, 1677 CrossRef CAS
 .
. - L. Ma, S. Liang, X. L. Liu, D. J. Yang, L. Zhou and Q. Q. Wang, Adv. Funct. Mater., 2015, 25, 898 CrossRef CAS
 .
. - J. Wang, Y. Yu and L. Zhang, Appl. Catal., B, 2013, 136, 112 CrossRef
 .
. - A. Fujishima, X. Zhang and D. Tryk, Surf. Sci. Rep., 2008, 63, 515 CrossRef CAS
 .
. - J. Kim, C. W. Lee and W. Choi, Environ. Sci. Technol., 2010, 44, 6849 CrossRef CAS PubMed
 .
.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were mixed and dissolved by stirring for 1 h. Subsequently, the mixture was loaded in a 100 mL Teflon-lined stainless steel autoclave. The autoclave was sealed and heated in a program-controlled oven at 180 °C for 10 h and then air-cooled to room temperature. The resultant products were separated by centrifugation, washed with distilled water and ethanol, and dried in a vacuum at 60 °C for one night. We also conducted a hydrothermal method for the synthesis of pure CdS microspheres using the same method as above in the absence of SrTiO3, which is reported in the literature.32
2) were mixed and dissolved by stirring for 1 h. Subsequently, the mixture was loaded in a 100 mL Teflon-lined stainless steel autoclave. The autoclave was sealed and heated in a program-controlled oven at 180 °C for 10 h and then air-cooled to room temperature. The resultant products were separated by centrifugation, washed with distilled water and ethanol, and dried in a vacuum at 60 °C for one night. We also conducted a hydrothermal method for the synthesis of pure CdS microspheres using the same method as above in the absence of SrTiO3, which is reported in the literature.32
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 5 min) to remove the photocatalyst particles. The concentration of aqueous CIP was determined with a spectrometer by measuring the absorbance at 277 nm.33 The photocatalytic degradation ratio (DR) was calculated by the following formula:
000 rpm, 5 min) to remove the photocatalyst particles. The concentration of aqueous CIP was determined with a spectrometer by measuring the absorbance at 277 nm.33 The photocatalytic degradation ratio (DR) was calculated by the following formula:







.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.




