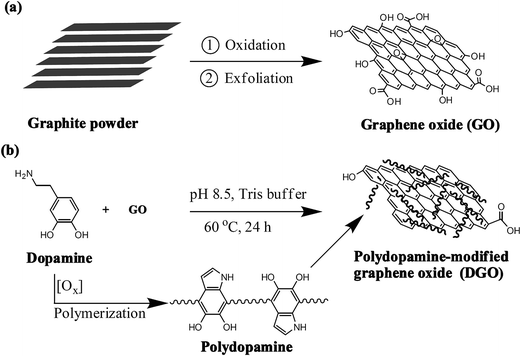
Nanocomposite membranes modified by graphene-based materials for anion exchange membrane fuel cells
Zhengyuan Luo,
Yujiao Gong,
Xiaofeng Liao,
Yongjun Pan and
Hongwei Zhang*
College of Materials Science and Engineering, Wuhan Textile University, Wuhan, 430073, PR China. E-mail: hanqiujiang@163.com; Fax: +86-27-59367580; Tel: +86-27-59367580
First published on 27th January 2016
Abstract
Graphene oxide (GO) is synthesized through the oxidation reaction of graphite with strong oxidants and GO modified by dopamine (DGO) is also prepared via in situ self-polymerization of dopamine to form a polydopamine coating on the surface of GO. The GO or DGO is blended with chloromethylated polysulfone (CMPSF) in dimethylacetamide to prepare composite anion-exchange membranes (AEMs) by solution casting, followed by quaternization and alkalization. The synthesis of GO and DGO is confirmed by Fourier transform infrared attenuated total reflection. The properties of AEMs including their water uptake, thermal stability, mechanical property and ionic conductivity are investigated. Compared with the plain quaternized polysulfone membrane, the composite membranes containing 1% GO or 0.5% DGO display higher water uptakes, stronger tensile strengths, a bigger elongation rate at break and better alkaline solution stability. The ionic conductivities of them are 1.08 × 10−2 S cm−1 and 1.07 × 10−2 S cm−1 at 60 °C, respectively. Moreover, 86.9% and 76% of the two values can be maintained after the two composite membranes are treated in 1 M NaOH solution for 120 h at 50 °C.
1. Introduction
As a clean and environmentally friendly potential power source of the future, fuel cells have been drawing great attention in the past several decades. Among them, fuel cells using proton-exchange membranes (PEMFCs), which are considered as alternative power supplies in mobile phones, laptops and other small household energy devices, are still not used for commercial applications due to several unresolved problems including high cost and insufficient durability.1As a consequence, fuel cells using anion exchange membranes (AEMFCs, analogs of PEMFCs) are becoming a new research hotspot for fuel cells in recent years because of their advantages in alkaline conditions, faster electro-kinetics, increased material stability, and broader choice of fuels.2
An AEMFC with a maximum power density of 823 mW cm−2 (ref. 3) has been reported and another AEMFC with a lifetime of nearly 5000 h (ref. 4) has also been reported. These single values are comparable to those of PEMFCs, but the comprehensive performances of AEMFCs are still inferior to those of PEMFCs. Since the AEM is one of the crucial components of AEMFCs and its performance determines the performance of the AEMFC to a very great extent, the studies of AEMs are of high priority. At least six categories of AEMs have been explored, such as AEMs derived from Nafion precursors with sulfonyl fluoride groups,5 AEMs prepared by grafting technologies,6,7 AEMs based on functionalized commercial polymers,8–10 AEMs prepared by newly-synthesized polymers,11–17 AEMs containing heterogeneous compositions18–20 and AEMs with functional groups different from quaternary ammonium.21–23 Although some AEMs having cleverly designed structures displayed exciting results such as high ionic conductivities comparable to that of Nafion,14–17 the performance and long lifetime of most AEMs are still lower than that of the Nafion membrane. As a consequence, further studies are needed to look for AEMs with high performance and long lifetime.
As a two-dimensional nano-material, graphene has potential applications in various fields due to its unique electronic properties, facile synthesis, and ease of functionalization.24 Graphene-based materials have been utilized to prepare proton-exchange membranes for PEMFCs and enhanced performances have been achieved.25,26
In this work, graphene oxide (GO) and GO modified by dopamine (DGO) are prepared. Then, composite membranes consisting of chloromethylated polysulfone (CMPSF) and GO or DGO are converted to AEMs via quaternization and alkalization. The AEMs are characterised with the help of structural analysis, mechanical analysis, morphological analysis, water uptake and ionic conductivity measurements. The effects of GO and DGO on the properties of AEMs are synthetically studied.
2. Experimental
2.1 Synthesis of GO and DGO
GO was synthesized from natural graphite powder according to the modified Hummer’s method.27 After graphite powder (5 g) and sodium nitrate (NaNO3, 2.5 g) were blended in a round-bottom flask, concentrated H2SO4 (100 mL) was slowly added and the mixture stirred for 30 min at 0 °C in an ice bath. Potassium permanganate (KMnO4, 15 g) was very slowly added into the flask under stirring and the reaction temperature of the mixture was kept below 10 °C in the ice-bath during this period. Then the mixture was further stirred for 2 h at room temperature. Next, the reaction temperature was increased to 35 °C and the mixture stirred for 30 min. After that, deionized water (100 mL) was added to dilute the mixture drop by drop, producing a large exotherm to 95 °C. The flask was heated in order to maintain the reaction temperature at 95 °C for 30 min in an oil bath. Additional deionized water (50 mL) and 30% H2O2 (25 mL) were added and stirred for 15 min. After 10% hydrochloric acid (HCl, 50 mL) was added to the resulting mixture and the mixture stirred for 5 min, the product was purified in succession with centrifugation and washing with water several times. The wet product, about 1.5 g, was washed by 50 mL dimethylacetamide (DMAc) to replace water and re-dispersed in 100 mL DMAc, followed by ultrasonic treatment in a 100 W sonic bath for 2 h to obtain the GO suspension in DMAc. The other wet product was re-dispersed in 500 mL water and bath-sonicated for 2 h to obtain the GO suspension in water.The DGO was prepared through in situ self-polymerization of dopamine in GO aqueous solution. The procedure was as follows:28 0.5 g of dopamine hydrochloride was dissolved in 25 mL water and added to 55 mL GO aqueous solution (about 0.5 g GO) at room temperature. The pH value of the reaction solution was controlled at 8.5 by Tris-buffer solution. The reaction mixture was stirred at 60 °C for 24 h. After cooling to room temperature, the mixture was purified in succession with centrifugation and washing with water at least three times. Then the collected product was washed with 50 mL dimethylacetamide (DMAc) to replace water and re-dispersed in 100 mL DMAc to form the DGO suspension in DMAc.
2.2 Synthesis of CMPSF
CMPSF was synthesized according to the literature.29 Briefly, the procedure was as follows: 11 g polysulfone (Udel P3500, Solvay) was dissolved in 300 mL dichloromethane and then treated with 16 mL chloromethyl ether (CME) in the presence of 3 mL anhydrous tin chloride at 30 °C for 30 min to obtain the CMPSF. Lastly, the mixture was treated in 3 L ethanol to obtain the CMPSF precipitate, followed by filtration, washing with water and evaporation.2.3 Fabrication of AEMs
At first, CMPSF was dissolved in dimethylacetamide (DMAc) to make a 15% (w/v) solution, followed by stirring, and filtration. Then the GO or DGO suspension of preassigned content (if necessary) was added to cast the films. Before casting, N,N,N′,N′-tetramethylethylenediamine (TMEDA) was added into the casting solution as a crosslinking agent (0.3 mL/10 g CMPSF casting solution) to form partial crosslinks in membranes. Then the film was dried under vacuum at 80 °C for 24 h. The resultant membrane was immersed in 30 wt% trimethylamine (TMA) solution for 24 h to induct quaternary groups into the membrane. Thereafter, the membrane was put into 1 M KOH solution for another 24 h. Lastly, the resultant membrane was washed several times with distilled water and naturally dried to obtain AEMs under an ambient environment to avoid any great shrinkage with water loss. The control AEM without fillers was designated as the QPSF membrane. Depending on the content of GO or DGO, the composite AEMs were designated as the QPSF/x% GO (or DGO) membrane, where x = 0.5, 1 and 2.2.4 Structure characterizations
Fourier transform infrared attenuated total reflection (FTIR-ATR) spectra of GO, DGO and AEMs were recorded using a VERTEX70 spectrometer. The thermal analysis of the AEMs was performed by using a NETZSCH Instrument. The surface morphology of samples was examined through a scanning electron microscopy (SEM, JEOL JSM-6510). After the sample was dried, it was vacuum-deposited with a thin Au film for the SEM examination. The morphology of the samples was also examined through a transmission electron microscope (TEM, Tecnai G2 20). The dyed sample was embedded in epoxy resin and sectioned using a microtome to yield a 100 nm thick sample which was placed on copper grids for TEM images.2.5 Ion-exchange capacity
IEC of AEMs was determined using the back titration method. A weighted dry AEM sample was immersed in 100 mL of 1 M NaCl solution for 48 h at room temperature. Then the NaCl solution was titrated with 0.1 M HCl solution. Based on the titration results, the IEC (mmol g−1) of the membrane was calculated as follows:
 | (1) |
2.6 Water uptake
All membranes were vacuum dried at 100 °C before water uptake testing. Then the AEMs were soaked in deionized water for 24 h at a certain temperature. Weights of dry and wet membranes were measured. The water uptake content was calculated by:
 | (2) |
The lengths of the dry and wet samples were measured. The swelling ratio was the average of the two measurements with an error within ±3.0% and calculated from the length of films by:
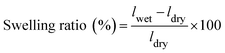 | (3) |
2.7 Mechanical properties
Tensile strength was measured by using an INSTRWN5566 Mechanical Testing Machine. The membrane samples were cut to 0.5 cm × 5.0 cm and humidified in 100% relative humidity (RH) for 24 h at room temperature before testing. The samples were examined at an elongation rate of 10 mm min−1. The tensile strength was calculated with the following equation:
 | (4) |
2.8 Hydroxide ion conductivity
The ionic conductivity (σ) of the membranes was measured by the two-probe AC method from 1 Hz to 500 KHz and 10 mV AC perturbation on an Autolab work station. A sample with a size of 15 mm × 15 mm was placed in an open, temperature controlled cell where it was clamped between two platinum electrodes. Specimens were soaked in deionized water for at least 48 h prior to the test. The impedance measurement was performed in water with 100% relative humidity (RH) at the desired temperature. The σ of the membrane in the direction perpendicular to the surface was calculated from the impedance data, using the formula:
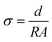 | (5) |
Based on the Arrhenius relationship between ionic conductivity and temperature:
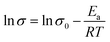 | (6) |
The apparent activation energy (Ea) of ionic conductivity was calculated according to the following formula:
| Ea = −b × R | (7) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) σ against 1000/T.
σ against 1000/T.
2.9 Alkaline solution stability
The alkaline solution stability of the membranes was evaluated by ionic conductivity after the membrane was immersed in 1 M NaOH solution at 50 °C for 120 h.3. Results and discussion
3.1 GO and DGO synthesis
The procedure used to synthesize GO and DGO is briefly illustrated in Scheme 1. In fact, the synthesis of DGO included two reactions. One was the self-polymerization of dopamine, the other was the simultaneous reduction reaction of GO. Because dopamine was prone to spontaneous oxidative polymerization and readily adhered to material surfaces, GO covered with a thin polydopamine (PDA) coating could be prepared through the in situ self-polymerization of dopamine in the presence of GO. When GO was well-dispersed in a distilled water medium, the GO solution had a light brown color, and the brown color could be maintained for the GO film whether it was in the QPSF/1% GO membrane or in the CMPSF/1% GO casting solution (Fig. 1a, c and e). After GO was modified by dopamine, DGO appeared a complete opaque black color because GO was partly reduced. Furthermore, the black color was also kept whether in the QPSF/1% DGO membrane or in the CMPSF/1% DGO casting solution (Fig. 1b, d and f). The distribution of GO and DGO in the composite membranes were further characterized by SEM and TEM. From Fig. 2a and c, it could be observed that the GO was well-dispersed in the QPSF/1% GO membrane; the thickness of GO was in the nanoscale. But the DGO displayed some agglomerates in Fig. 2b and d, which resulted in a bigger size of the DGO particles. The difference might be explained by the electrostatic interactions between quaternary ammonium groups and carboxylic acid groups on GO which promoted the dispersion of GO in the QPSF/1% GO membrane as well as repelling interactions between quaternary ammonium groups and amine groups on PDA, which facilitated the agglomerates of DGO in the QPSF/1% DGO membrane. | ||
| Fig. 1 Digital photos of (a) GO, (b) DGO, (c) CMPSF/1% GO and (d) CMPSF/1% DGO casting solutions, and (e) QPSF/1% GO and (f) QPSF/1% DGO composite membranes. | ||
 | ||
| Fig. 2 SEM images of (a) QPSF/1% GO and (b) QPSF/1% DGO membranes, and TEM images of (c) QPSF/1% GO and (d) QPSF/1% DGO membranes. | ||
3.2 FTIR-ATR analysis
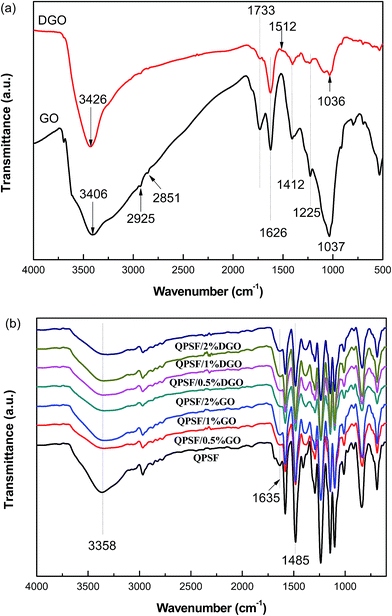
In order to identify the functional groups on the surface of GO and DGO, FTIR-ATR spectra of them were recorded and are shown in Fig. 3a. As observed from the FTIR-ATR spectrum of GO, the characteristic absorption peaks appeared at 1733, 1626, 1412, 1225 and 1037 cm−1, which could be associated with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration in the carboxylic acid and carbonyl moieties, conjugated C
O stretching vibration in the carboxylic acid and carbonyl moieties, conjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of unoxidized graphite domains, O–H deformation vibration of the –COOH groups, C–OH stretching vibration of the –COOH groups and C–O stretching vibrations of epoxy or alkoxy groups, respectively. Besides, the broad and intensive absorption peak centered at 3406 cm−1 was attributed to the O–H stretching vibrations of water adsorbed inside the GO, hydroxyl and carboxylic acid groups. The absorption peaks around 2925 and 2851 cm−1 correspond to the stretching vibrations of aliphatic sp3 C–H. These results were consistent with the literature,30,31 suggesting that GO had been successfully synthesized through the oxidation of graphite.
C of unoxidized graphite domains, O–H deformation vibration of the –COOH groups, C–OH stretching vibration of the –COOH groups and C–O stretching vibrations of epoxy or alkoxy groups, respectively. Besides, the broad and intensive absorption peak centered at 3406 cm−1 was attributed to the O–H stretching vibrations of water adsorbed inside the GO, hydroxyl and carboxylic acid groups. The absorption peaks around 2925 and 2851 cm−1 correspond to the stretching vibrations of aliphatic sp3 C–H. These results were consistent with the literature,30,31 suggesting that GO had been successfully synthesized through the oxidation of graphite.
In the spectrum of DGO, it was clearly revealed that the intensities of these absorption peaks relative to the oxygen-containing functional groups were significantly weakened, indicating that GO was partly reduced during the in situ polymerization of dopamine. The typical absorption peaks at 3426 and 1512 cm−1 were assigned to the catechol O–H stretching vibrations and N–H bending vibrations of PDA. But the typical absorption peak at 1626 cm−1, which originated from the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations of the phenol structure in PDA, was indistinguishable because of overlap with the intensive peak at 1626 cm−1 of GO. The self-polymerization of dopamine was also verified by the absence of an absorption peak at 1345 cm−1 arising from the CH2 bending vibrations in dopamine. These results were in agreement with the literature,28,32 confirming that the coating of GO with thin PDA to form DGO was successful.
C stretching vibrations of the phenol structure in PDA, was indistinguishable because of overlap with the intensive peak at 1626 cm−1 of GO. The self-polymerization of dopamine was also verified by the absence of an absorption peak at 1345 cm−1 arising from the CH2 bending vibrations in dopamine. These results were in agreement with the literature,28,32 confirming that the coating of GO with thin PDA to form DGO was successful.
FTIR-ATR spectra of AEMs are presented in Fig. 3b. It is obvious that the characteristic absorption peaks of GO and DGO are indescribable due to the low contents of them in the composite AEMs. The absorption peaks at 1485 and 1635 cm−1 were generated by the stretching vibration of quaternary ammonium groups, suggesting the successful quaternization.33
3.3 Thermal analysis
The thermal decomposition curves of QPSF, QPSF/1% GO and QPSF/1% DGO membranes are plotted in Fig. 4. Obviously, the weight losses of them could be divided into three steps. The first step from room temperature to 164.3 °C was caused by the removal of the physically and chemically absorbed water in the membranes. The second step from 165.6 °C to 383 °C was induced by the degradation of the quaternary ammonium groups and the breakage of crosslinks in the membranes. The third step above 373.1 °C was attributed to the decomposition of the polymer backbones. Compared with the QPSF membrane, QPSF/1% GO and QPSF/1% DGO membranes showed faster weight losses from room temperature to 383 °C. This phenomenon could be explained by two aspects. On the one side, the hydrophilic nano-sheets doped in membranes facilitated the water absorption of the composite membranes. On the other side, the incorporation of nano-sheets with great surfaces might partly disturb the crosslinking in the composite membranes, which led to an easier decomposition of some compositions. The decomposition temperatures of quaternary ammonium groups in these membranes indicated that they could meet the operation temperature of AEMFCs.3.4 Mechanical property
In order to simulate the operation condition of AEMs, all the membranes were balanced in water vapour at 25 °C for 24 h before testing. The stress–strain curves of humidified AEMs are listed in Table 1. The QPSF demonstrated a tensile strength of about 12 MPa and an elongation rate at break of 17.3% because of the plasticization of water molecules. The introduction of the GO sheets produced two contrary influences. One was to interrupt the cross-links in part, which weakened the interactions of the polymer chains. The other was to form ionic cross-links between quaternary ammonium groups and carboxylic acid groups, which enhanced the electrostatic interactions in the QPSF/GO membranes. Evidently, the latter influence was dominate in the QPSF/GO membranes and the tensile strength of them increased with the increase of the content of GO. But the QPSF/1% GO membrane exhibited the longest elongation rate at break of 33% with a tensile strength of 13.1 MPa. Similarly, the DGO sheets could interfere with the cross-links in the QPSF/DGO membranes. Moreover, the coating of PDA on the DGO could improve the tenacity through a slight slippage in the interfaces. As a consequence, the tensile strength of the QPSF/DGO membranes decreased with the increase of the content of DGO, but the elongation rate at break increased with the increase of the content of DGO. The lowest elongation rate of them at break was 33.1%.| Membranes | Thickness (μm) | IEC (meq g−1) | Water uptake (%) | Swelling ratio (%) | Tensile strength (MPa) | Maximum elongation (%) | ||
|---|---|---|---|---|---|---|---|---|
| 60 °C | 95 °C | 60 °C | 95 °C | |||||
| QPSF/0.5% GO | 83 | 1.21 | 23.44 | 30.69 | 12.38 | 15.64 | 12.07 | 24.99 |
| QPSF/1% GO | 89 | 1.17 | 24.75 | 41.68 | 12.61 | 16.35 | 13.31 | 32.94 |
| QPSF/2% GO | 82 | 1.19 | 31.41 | 57.10 | 13.42 | 17.12 | 13.62 | 16.05 |
| QPSF/0.5% DGO | 78 | 1.23 | 29.10 | 40.15 | 13.50 | 18.07 | 10.03 | 32.96 |
| QPSF/1% DGO | 81 | 1.20 | 36.87 | 45.23 | 14.23 | 19.86 | 8.97 | 43.47 |
| QPSF/2% DGO | 90 | 1.17 | 41.69 | 54.92 | 17.05 | 21.69 | 7.21 | 44.43 |
| QPSF | 85 | 1.23 | 22.80 | 27.54 | 4.12 | 7.31 | 12.03 | 17.04 |
3.5 Water uptake
Moderate water uptake in AEMs was necessary for a high ionic conductivity. The water uptakes and swelling ratios of AEMs are also listed in Table 1. The water uptakes of all AEMs rose with the increase of temperature. But QPSF/GO and QPSF/DGO membranes revealed different water uptake behaviors. The water uptake of QPSF/GO membranes exhibited a slightly ascending trend along with the increase of the content of GO, whereas the water uptake curves of QPSF/DGO membranes showed remarkably incremental trends accompanied with the increase of the content of DGO. The difference between them was also caused by the same reasons explained above in section 3.4. The QPSF/1% GO and QPSF/0.5% DGO membranes showed modest water uptakes even at 95 °C and water uptakes of 24.75% and 29.1% at 60 °C, respectively. Compared with the water uptakes, the swelling ratios of the two kinds of hybrid AEMs showed similar trends. The low values of swelling ratios suggested that they had good dimensional stability.3.6 Ionic conductivity and alkaline solution stability
The ionic conductivities of all AEMs at different temperatures are plotted in Fig. 5a. All of them increased with the increasing temperature. The values of the apparent activation energies (Ea) for the OH− anion transfer revealed that OH− ions were transported in these AEMs via a Grotthuss-type mechanism. It was clear that the ionic conductivities of the composite membranes were lower than that of the QPSF membrane. For QPSF/GO membranes, the ionic conductivities were gradually reduced with the increase of the content of GO due to the tortuous ionic channels formed by GO. Furthermore, the Ea of ionic conductivity of the QPSF/GO membranes increased from 14.41 kJ mol−1 to 17.89 kJ mol−1 with the increase of the content of GO, which meant that the increase of the transfer barrier for OH− anions was due to the tortuous ionic channels. Whereas for QPSF/DGO membranes, the ionic conductivities also decreased step by step with the increase of the content of DGO because of the collective effects of the low efficiency of DGO for OH− anion transfer and tortuous ionic channels. The similar Ea values of the QPSF/DGO membranes suggested a nearly uninfluential transfer barrier for the OH− anions owing to the greatly increased water uptake. The QPSF/1% GO and QPSF/0.5% DGO membranes displayed ionic conductivities of 1.08 × 10−2 S cm−1 and 1.07 × 10−2 S cm−1 at 60 °C, respectively. Although the ionic conductivities of QPSF/1% GO and QPSF/0.5% DGO membranes could meet the basic requirements for AEMs, they were still lower than those values reported in the literature for different AEMs, which are listed in Table 2. Hence, further investigations of this kind of AEM are needed. | ||
| Fig. 5 (a) Ionic conductivity of all AEMs at different temperatures and (b) the alkaline solution stability. | ||
| Membrane material | Functional groups | IEC (meq g−1) | Conductivity (×10−2 S cm−1) | Reference |
|---|---|---|---|---|
| a PTMA = bis(phenyltrimethylammonium).b QPMBV = imidazolium-functionalized poly(ether ether ketone).c PVBC-g-PAES = quaternary ammonium poly(2,6-dimethyl-phenylene oxide).d QPE = quaternized multiblock copolymer.e TPQPOH = methylene quaternaryphosphonium-hydroxide.f QPEN = quaternized benzylmethyl-containing poly(arylene ether nitrile). | ||||
| QPSF/1% GO | Quaternary ammonium | 1.17 | 1.08 (60 °C) | This study |
| QPSF/0.5% DGO | Quaternary ammonium | 1.23 | 1.07 (60 °C) | This study |
| PTMA functionalized PSFa | Quaternary ammonium | 1.74 | 5.8 (80 °C) | 7 |
| PEEK-ImOHb | Imidazolium | 2.03 | 5.2 (20 °C) | 8 |
| Quaternized poly(ether sulfone) | Quaternary ammonium | 1.16 | 2.23 (25 °C) | 9 |
| QPPOc | Quaternary ammonium | 3.20 | 4.0 (25 °C) | 10 |
| Polyethylene block copolymer | Quaternary ammonium | 1.92 | 7.3 (60 °C) | 14 |
| QPEd | Quaternary ammonium | 1.93 | 12.6 (60 °C) | 17 |
| Quaternary phosphonium polysulfone | TPQPOHe | 1.17 | 4.50 (20 °C) | 34 |
| QPENf | Quaternary ammonium | 2.91 | 5.60 (60 °C) | 35 |
The ionic conductivities of QPSF, QPSF/1% GO and QPSF/0.5% DGO membranes at 60 °C after the alkaline solution stability test are presented in Fig. 5b. The residual ionic conductivities of them were 66.1%, 86.9% and 76.0%, respectively. These results exhibited that the incorporation of GO or DGO could enhance the alkaline solution stability of AEMs. Moreover, the tensile strengths and elongation rates of QPSF/1% GO and QPSF/0.5% DGO membranes, which were treated in 1 M NaOH solution at 50 °C for 120 h, were 11.83 MPa and 30.62%, and 9.16 MPa and 29.63%, respectively, indicating a rather high mechanical stability.
4. Conclusions
Here, GO was synthesized by the oxidation of graphite powders and DGO was also prepared by in situ self-polymerization of dopamine successfully. Two graphene-based materials were used to improve the properties of AEMs. Both QPSF/GO and QPSF/DGO membranes showed an improved water uptake and tensile strength, increased elongation rate at break, enhanced alkaline solution stability and acceptably reduced ionic conductivities. The QPSF/1% GO and QPSF/0.5% DGO membranes displayed ionic conductivities of 1.08 × 10−2 S cm−1 and 1.07 × 10−2 S cm−1 at 60 °C, respectively. The corresponding ionic conductivities were still maintained at 86.9% and 76.0% at 60 °C after the alkaline solution stability test, implying that they could meet the requirements for AEMs.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (51503161).References
- H. Zhang and P. K. Shen, Recent Development of Polymer Electrolyte Membranes for Fuel Cells, Chem. Rev., 2012, 112, 2780–2832 CrossRef CAS PubMed.
- J. R. Varcoe, P. Atanassov, D. R. Dekel, A. M. Herring, M. A. Hickner and P. A. Kohl, et al., Anion-exchange membranes in electrochemical energy systems, Energy Environ. Sci., 2014, 7, 3135–3191 CAS.
- M. Mamlouk, J. A. Horsfall, C. Williams and K. Scott, Radiation grafted membranes for superior anion exchange polymer membrane fuel cells performance, Int. J. Hydrogen Energy, 2012, 37, 11912–11920 CrossRef CAS.
- Y. S. Kim, Resonance-Stabilized Anion Exchange Polymer Electrolytes, 2012, http://www.hydrogen.energy.gov/pdfs/review12/fc043_kim_2012_p.pdf.
- H. L. S. Salerno, F. L. Beyer and Y. A. Elabd, Anion exchange membranes derived from nafion precursor for the alkaline fuel cell, J. Polym. Sci., Part B: Polym. Phys., 2012, 50, 552–562 CrossRef CAS.
- Z. Xia, S. Yuan, G. Jiang, X. Guo, J. Fang and L. Liu, et al., Polybenzimidazoles with pendant quaternary ammonium groups as potential anion exchange membranes for fuel cells, J. Membr. Sci., 2012, 390–391, 152–159 CrossRef CAS.
- N. Li, Q. Zhang, C. Wang, Y. M. Lee and M. D. Guiver, Phenyltrimethylammonium Functionalized Polysulfone Anion Exchange Membranes, Macromolecules, 2012, 45, 2411–2419 CrossRef CAS.
- X. Yan, S. Gu, G. He, X. Wu and J. Benziger, Imidazolium-functionalized poly(ether ether ketone) as membrane and electrode ionomer for low-temperature alkaline membrane direct methanol fuel cell, J. Power Sources, 2014, 250, 90–97 CrossRef CAS.
- S. S. Koilpillai and S. Dharmalingam, A novel quaternized poly(ether sulfone) membrane for alkaline fuel cell application, Int. J. Energy Res., 2015, 39, 317–325 CrossRef CAS.
- N. Li, L. Wang and M. Hickner, Cross-linked comb-shaped anion exchange membranes with high base stability, Chem. Commun., 2014, 50, 4092–4095 RSC.
- Z. Zhao, F. Gong, S. Zhang and S. Li, Poly(arylene ether sulfone)s ionomers containing quaternized triptycene groups for alkaline fuel cell, J. Power Sources, 2012, 218, 368–374 CrossRef CAS.
- P. Yu Xu, K. Zhou, G. Lu Han, Q. Gen Zhang, A. Mei Zhu and Q. Lin Liu, Fluorene-containing poly(arylene ether sulfone)s as anion exchange membranes for alkaline fuel cells, J. Membr. Sci., 2014, 457, 29–38 CrossRef.
- S. Zhang, C. Li, X. Xie and F. Zhang, Novel cross-linked anion exchange membranes with diamines as ionic exchange functional groups and crosslinking groups, Int. J. Hydrogen Energy, 2014, 39, 13718–13724 CrossRef CAS.
- Y. Li, Y. Liu, A. M. Savage, F. L. Beyer, S. Seifert and A. M. Herring, et al., Polyethylene-Based Block Copolymers for Anion Exchange Membranes, Macromolecules, 2015, 48, 6523–6533 CrossRef CAS.
- X. Li, Q. Liu, Y. Yu and Y. Meng, Synthesis and properties of multiblock ionomers containing densely functionalized hydrophilic blocks for anion exchange membranes, J. Membr. Sci., 2014, 467, 1–12 CrossRef CAS.
- D. W. Seo, Y. D. Lim, M. A. Hossain, S. H. Lee, H. C. Lee and H. H. Jang, et al., Anion conductive poly(tetraphenyl phthalazine ether sulfone) containing tetra quaternary ammonium hydroxide for alkaline fuel cell application, Int. J. Hydrogen Energy, 2013, 38, 579–587 CrossRef CAS.
- M. Tanaka, K. Fukasawa, E. Nishino, S. Yamaguchi, K. Yamada and H. Tanaka, et al., Anion Conductive Block Poly(arylene ether)s: Synthesis, Properties, and Application in Alkaline Fuel Cells, J. Am. Chem. Soc., 2011, 133, 10646–10654 CrossRef CAS PubMed.
- X. Li, Y. Yu and Y. Meng, Novel Quaternized Poly(arylene ether sulfone)/Nano-ZrO2 Composite Anion Exchange Membranes for Alkaline Fuel Cells, ACS Appl. Mater. Interfaces, 2013, 5, 1414–1422 CAS.
- J. Fan, H. Zhu, R. Li, N. Chen and K. Han, Layered double hydroxide-polyphosphazene-based ionomer hybrid membranes with electric field-aligned domains for hydroxide transport, J. Mater. Chem. A, 2014, 2, 8376–8385 CAS.
- Y. Zhao, H. Yu, D. Xing, W. Lu, Z. Shao and B. Yi, Preparation and characterization of PTFE based composite anion exchange membranes for alkaline fuel cells, J. Membr. Sci., 2012, 421–422, 311–317 CrossRef CAS.
- X. Lin, M. Gong, Y. Liu, L. Wu, Y. Li and X. Liang, et al., A convenient, efficient and green route for preparing anion exchange membranes for potential application in alkaline fuel cells, J. Membr. Sci., 2013, 425–426, 190–199 CrossRef CAS.
- S. Sharma, M. Dinda, C. R. Sharma and P. K. Ghosh, A safer route for preparation of anion exchange membrane from inter-polymer film and performance evaluation in electrodialytic application, J. Membr. Sci., 2014, 459, 122–131 CrossRef CAS.
- J. Miyake, K. Fukasawa, M. Watanabe and K. Miyatake, Effect of ammonium groups on the properties and alkaline stability of poly(arylene ether)-based anion exchange membranes, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 383–389 CrossRef CAS.
- C. Hu, L. Song, Z. Zhang, N. Chen, Z. Feng and L. Qu, Tailored graphene systems for unconventional applications in energy conversion and storage devices, Energy Environ. Sci., 2015, 8, 31–54 CAS.
- Y. He, J. Wang, H. Zhang, T. Zhang, B. Zhang and S. Cao, et al., Polydopamine-modified graphene oxide nanocomposite membrane for proton exchange membrane fuel cell under anhydrous conditions, J. Mater. Chem. A, 2014, 2, 9548–9558 CAS.
- Y. He, C. Tong, L. Geng, L. Liu and C. Lü, Enhanced performance of the sulfonated polyimide proton exchange membranes by graphene oxide: Size effect of graphene oxide, J. Membr. Sci., 2014, 458, 36–46 CrossRef CAS.
- W. S. Hummers Jr and R. E. Offeman, Preparation of graphitic oxide, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef.
- W. Lee, J. U. Lee, B. M. Jung, J.-H. Byun, J.-W. Yi and S.-B. Lee, et al., Simultaneous enhancement of mechanical, electrical and thermal properties of graphene oxide paper by embedding dopamine, Carbon, 2013, 65, 296–304 CrossRef CAS.
- X. Liao, L. Ren, D. Chen, X. Liu and H. Zhang, Nanocomposite membranes based on quaternized polysulfone and functionalized montmorillonite for anion-exchange membranes, J. Power Sources, 2015, 286, 258–263 CrossRef CAS.
- L.-B. Zhang, J.-Q. Wang, H.-G. Wang, Y. Xu, Z.-F. Wang and Z.-P. Li, et al., Preparation, mechanical and thermal properties of functionalized graphene/polyimide nanocomposites, Composites, Part A, 2012, 43, 1537–1545 CrossRef CAS.
- S. Mallakpour, A. Abdolmaleki and S. Borandeh, Covalently functionalized graphene sheets with biocompatible natural amino acids, Appl. Surf. Sci., 2014, 307, 533–542 CrossRef CAS.
- L. Zhu, Y. Lu, Y. Wang, L. Zhang and W. Wang, Preparation and characterization of dopamine-decorated hydrophilic carbon black, Appl. Surf. Sci., 2012, 258, 5387–5393 CrossRef CAS.
- L. Zeng and T. S. Zhao, High-performance alkaline ionomer for alkaline exchange membrane fuel cells, Electrochem. Commun., 2013, 34, 278–281 CrossRef CAS.
- S. Gu, R. Cai, T. Luo, K. Jensen, C. Contreras and Y. S. Yan, Quaternary Phosphonium-Based Polymers as Hydroxide Exchange Membranes, ChemSusChem, 2010, 3, 555–558 CrossRef CAS PubMed.
- A. N. Lai, L. S. Wang, C. X. Lin, Y. Z. Zhuo, Q. G. Zhang and A. M. Zhu, et al., Benzylmethyl-containing poly(arylene ether nitrile) as anion exchange membranes for alkaline fuel cells, J. Membr. Sci., 2015, 481, 9–18 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |