Self-assembled nc-Si-QD/a-SiC thin films from planar ICP-CVD plasma without H2-dilution: a combination of wide optical gap, high conductivity and preferred 〈220〉 crystallographic orientation, uniquely appropriate for nc-Si solar cells
Debajyoti Das* and
Debjit Kar
Nano-Science Group, Energy Research Unit, Indian Association for the Cultivation of Science, Jadavpur, Kolkata – 700 032, India. E-mail: erdd@iacs.res.in; Fax: +91-33-24732805
First published on 17th December 2015
Abstract
Spontaneous miniaturization of self-assembled nc-Si-QDs with preferred 〈220〉 crystallographic orientation and a rapid synthesis of superior quality nc-Si-QD/a-SiC thin films with enhanced crystallinity, maintaining a combination of wide optical gap and simultaneous high electrical conductivity, has been attained from (SiH4 + CH4)-plasma, without any additional H2-dilution in inductively coupled plasma CVD. The optical gap widens due to the Si–C bonds in the amorphous network and the increased quantum confinement effect on the miniaturized Si-QDs. CH4 acts as the dual source of both C and atomic-H. High density of atomic-H available in the planar ICP promotes simultaneously the Si crystallization and its growth along the thermodynamically favored 〈220〉 crystallographic orientation, thereby contributing easier electrical transport, particularly at devices in superstrate configuration. After imminent doping, these could be efficiently utilized as window layers in third generation all-silicon solar cells.
1. Introduction
In photovoltaic technologies, nano-crystalline silicon quantum dots (nc-Si-QDs) embedded in a dielectric matrix forms a material of particular interest for its versatile application as a window layer in all-Si low-cost third-generation solar cells. In attempting to achieve a suitable window layer, there are many reported materials, e.g. silicon carbide, silicon nitride and silicon oxide, both in amorphous and nano-crystalline form.1–9 Owing to the wide optical band gap and high doping efficiency, amorphous hydrogenated silicon carbide (a-SiC:H) has been extensively studied and also successfully employed as a window layer for solar cells in p–i–n configuration with amorphous silicon (a-Si:H) as the absorber i-layer. While increasing the optical band gap by higher carbon inclusion in the amorphous network, the electrical conductivity of the a-SiC:H becomes poorer, which stands against its efficient utilization in window layers.1,10,11 In this context, nc-Si-QDs embedded in a-SiC:H could be a better alternative. The widening of the optical gap is provided both by the carbonated amorphous network and the quantum confinement effect arising from the nc-Si-QDs, which in turn could retain the conductivity from available proportional nanocrystallinity.12 In addition to maintaining a wide optical gap while retaining high conductivity of the doped window layer, the presence of the nc-Si component could provide a better nucleation path for the growth of crystallites in the intrinsic nc-Si i-layer of the nc-Si solar cells, which would promote better stability due to its lower light-induced degradation.13–16Considering its utility in nc-Si solar cells there have been a number of initiatives and various available approaches for the growth of nc-Si-QDs, e.g., by plasma enhanced CVD (both capacitively and inductively coupled), hotwire CVD, microwave electron cyclotron resonance, helical wave CVD or magnetron sputtering.17–22 The inclusion of C in the amorphous network leads to a lowering in the degree of crystallinity and this trade-off relation becomes a perpetual hindrance for device application.11,23 Inductively coupled plasma CVD with very high density of plasma species, low plasma sheath potentials and excellent uniformity of the plasma parameters in the radial and axial directions can provide a high density of atomic hydrogen over the growing film surface, which could be instrumental in strategic promotion of crystallization in the silicon network even with the C-inclusion.6,9,13,24–27
In this context, we report on the formation of self-assembled nanocrystalline silicon quantum dots in amorphous silicon carbide matrix (nc-Si-QD/a-SiC) with a high deposition rate by inductively coupled plasma CVD from (SiH4 + CH4)-plasma without any hydrogen dilution,28 and retaining the crystallinity at a high magnitude even after inclusion of a significant amount of carbon into the network. Extensive studies on the effect of increasing C incorporation on the structural, optical and electrical properties of the nc-Si-QD/a-SiC films have been carried out. Finally, a possible physical mechanism has been projected on the observed properties and a comparative study has been discussed on the superiority of nc-Si-QD/a-SiC thin films from the perspective of available data in the contemporary literature.
2. Experimental
Thin films of nanocrystalline silicon quantum dots embedded in amorphous silicon carbide matrix (nc-Si-QD/a-SiC) have been synthesized in a low pressure Inductively Coupled Plasma assisted Chemical Vapor Deposition (ICP-CVD) system. Within the cubic plasma chamber (side length 30 cm) the heating element is inserted behind the substrate holder to provide a constant temperature of ∼300 °C during the film growth. The RF field (13.56 MHz) of 350 W has been feed through a low inductance (0.54 μH) four-antenna flat spiral copper tube coil.27,29 The RF antenna is separated from the vacuum region across the quartz window. Films are produced from 2 sccm SiH4 used as the precursor gas and varying the flow rate of CH4 from 0 to 35 sccm, as the source of C in the plasma, both being introduced through two separate circular gas rings placed sequentially between the substrate holder and the quartz plate. Samples were deposited on Corning® Eagle2000™ glasses, p-type (100) single crystal silicon wafers and carbon-coated Cu microscope grids (Pacific Grid-Tech, USA) for various structural, optical and electrical measurements.The Dektak 6M profilometer was used to measure the thickness of the films. The X-ray diffractometry was performed with a Cu-Kα X-radiation (λ = 1.5418 Å) source and Bragg diffraction set-up (Rich Seifert 3000P). Raman spectra were obtained at room temperature in a backscattering geometry by a Renishaw inVia Raman Microscope with excitation wavelength of 514 nm from air-cooled Ar+ laser source, maintaining a low power density ∼2 mW cm−2 to avoid local heating on the film surface. The various bonding structures (between Si, C and H) of the films were investigated from infrared spectroscopy in the range 400–4000 nm on a Perkin Elmer Spectrum 100 FTIR spectrometer. Transmission electron microscopy was performed using a JEOL JSM 2010 system operating at 200 kV. The optical density data of the samples were obtained from absorption and reflection measurements in the UV-visible region, using a double beam spectrophotometer (Hitachi 330, Japan). Electrical conductivity of the samples was measured with coplanar Al electrodes deposited by thermal evaporation at room temperature and the measurement was carried out in a vacuum of 5 × 10−6 Torr at room temperature. The conductivity was evaluated from the current measured by a Keithley 6517A electrometer.
3. Results
The nature of variation in the degree of crystallinity of the nc-Si-QD/a-SiC thin films has been investigated by X-ray diffraction studies, as a function of varying flow rate (F) of CH4 in the SiH4 plasma. The nc-Si-QD/a-Si thin film prepared at F = 0 sccm demonstrates significant crystallinity by the characteristic diffraction peaks at 2θ ∼ 28.3°, 47.1° and 56.6° corresponding to 〈111〉, 〈220〉 and 〈311〉 planes of crystalline silicon.30 At the initial inclusion of CH4 in the plasma with F = 2 sccm, although the crystallinity does not change much, gradual but significant variation in the nature of crystallinity has been identified on further increase in F. The crystallographic configuration grossly changes from 〈111〉 towards 〈220〉 preferential orientation, as shown in Fig. 1(a). However, for F > 20 sccm, the overall crystallinity starts decreasing. The nature of changes in the preferred crystallographic orientation with increasing F can be monitored from the variation in relative intensity of 〈220〉 to 〈111〉 peaks, I〈220〉/I〈111〉, shown in Fig. 1(b).The variation in the nature of Si–Si bonding, from crystalline towards amorphous, has been investigated by the normalized Raman spectra shown in Fig. 2(a). At F = 0 sccm, the spectrum shows an asymmetric sharp peak at ∼516 cm−1 corresponding to the predominant crystalline silicon phase, along with its elongated tail towards lower wavenumber as the signature of only a small fraction of accompanying amorphous components in the network. A highly crystalline nature of the pristine silicon thin films is the characteristic of ICP-CVD operating at optimum parametric conditions.27,29 However, interesting phenomena appear with the incorporation of carbon in the silicon network by increasing F. It has been shown in Fig. 2(a) that the nature of the Raman spectrum does not change significantly for a systematic increase in CH4 flow within the plasma up to F ≤ 25 sccm. However, on further increase (F > 25 sccm) a drastic change in the nature of the spectrum with a broad Gaussian peak at ∼480 cm−1 identifies a rapid growth in the amorphous component that turns to the formation of an amorphous-dominated silicon network at F = 35 sccm.
The degree of crystallinity of the films has been quantitatively characterized by deconvoluting each spectrum into three independent satellite components using a routine least-squares fit, after background subtraction. The corresponding three peaks are assigned as follows: (i) the Lorentzian peak of nano-crystalline silicon (nc-Si) at ∼516 cm−1, (ii) an intermediate Gaussian peak at 500–510 cm−1 due to ultra-nanocrystalline silicon (unc-Si) or grain-boundary region and, lastly, (iii) a broad Gaussian peak of amorphous silicon (a-Si) at lower Raman shift of 480 cm−1.31–34 A representative deconvolution of peaks has been shown in Fig. 2(b) and from the integrated area of the deconvoluted components the crystalline volume fraction (Fc) has been estimated, considering the unc-Si component as the part and portion of crystallinity.13
The remarkable result shown in the inset of Fig. 2(b) demonstrates a systematic enhancement in the crystalline volume fraction (XC) in the network from ∼76.9% to 83.4% for increasing F from 0 to 20 sccm, beyond which XC starts decreasing, followed by a drastic deterioration to 63.8% at F = 30 sccm. The results indicate the realization of C-induced promotion of crystallization of the Si-network effective for a limited amount of C incorporation under optimized parametric conditions with ICP-CVD.
The systematic peak shift of the nc-Si components towards lower wavenumber along with maintaining the virtually similar narrow line-width realized with increasing F up to 20 sccm, as shown in Fig. 2(c), might be the result of variations in the phonon confinement effect and therefore suggest regular reduction in the size of the quantum dots (Si-QD).30 For crystalline silicon (c-Si), the momentum conservation law restricts the excited phonons at the centre of the Brillouin zone in contributing to the first order Raman scattering process. However, with the reduction in size of the crystalline silicon quantum dots, a quantum effect arises in the scattering process. With non-validation of the conservation of momentum law in the Si-QDs, the phonons are dispersed over the Brillouin zone and start contributing in Raman spectra due to spatial confinement of phonons within the Si-QDs by the presence of structural defects at nano-crystalline boundaries. Strong confinement effects occur when the radius of the silicon nano-crystals approaches the Bohr's radius of silicon (∼5 nm) and below.
Accordingly, Raman spectra for the nc-Si peaks have been simulated considering the phonon confinement effect of Si-QDs along with its Gaussian size distribution. To depict the phonon confinement effect in the Raman spectra, a sine wave has been taken as the confinement function, which disappears outside the Si-QD boundary and the phonon dispersion curve has been taken as proposed by Paillard.35 The expression for the first order Raman spectrum is given as follows:
 | (1) |
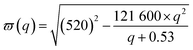 | (2) |
The abovementioned equations are appropriate for silicon nano-crystals with uniform size (d). However, in reality the Si-QDs must have a separate size distribution in each sample. Accordingly, the final expression for the Raman spectrum can be written as
| I(ω) ∝ ∫f(d)I(ω, d) | (3) |
 | (4) |
Systematic variation of crystallization in the nc-Si-QD/a-SiC films with the introduction of CH4 in the plasma has been studied by transmission electron microscopy (TEM). Fig. 3(a-i) represents the TEM micrograph of the pristine crystalline Si-network prepared at F = 0 sccm, wherein the crystalline Si-QDs, visible as deep dark spots, are randomly dispersed over a relatively bright and homogeneous a-Si network.36,37 The selected area electron diffraction (SAED) pattern of the films at the inset of Fig. 3(a-i) shows bright diffraction rings, which are identified as the 〈111〉, 〈220〉 and 〈311〉 planes of crystalline silicon. The presence of such planes has also been previously confirmed by the X-ray diffraction analysis. The high resolution TEM image in Fig. 3(a-ii) clearly depicts the lattice planes of the crystalline Si-QDs with distinct edges within the amorphous network.
In Fig. 3(b-i), the basic difference in the TEM image for F = 20 sccm, is the better contrast between the crystallites visible with relatively sharp edges. This might be due to the incorporation of C in the amorphous matrix and the Si-QDs have better contrast with the carbonated amorphous network due to larger difference in the density between the crystalline (nc-Si) and amorphous (a-SiC) components. The corresponding SAED image (inset of Fig. 3(b-i)) shows three prominent diffraction rings and spotted features, which identify higher degree of crystallinity. In the plain-view HR-TEM micrograph, the 〈220〉 crystal planes are relatively difficult to observe because of their specific geometrical projection on the two-dimensional plane, compared to its 〈111〉 counterpart. Spontaneous detection of the (220) lattice planes of c-Si on an arbitrarily chosen area of the sample, as shown in Fig. 3(b-ii), establishes the presence of a significant number of 〈220〉 oriented nc-Si-QDs within the a-SiC matrix prepared at F = 20 sccm. It is to be noted that for this nc-Si-QD/a-SiC film, a significantly high magnitude of I〈220〉/I〈111〉 ≥ 2 has been demonstrated by the XRD studies, identifying 〈220〉 preferential orientation. For further increase in F, however, the major change arises in the reduced size of the Si-QDs and their gradual amorphization.
The bonding configurations between Si, C and H have been investigated by infrared spectroscopy, performed on the films deposited on Si-wafers. The spectra have been corrected for the substrate absorption and normalized by the film thickness. The absorption spectra of the films have major peaks mainly in two spectral ranges around ∼550–950 cm−1 and 1900–2100 cm−1; the first range corresponds to the Si–H wagging and Si–C stretching mode absorptions, whereas the second range arises due to the stretching mode vibration of silicon hydrides (not shown here). Fig. 4(a) shows the absorption spectra for films prepared at various flow rates of CH4 in the lower wavenumber range. The pristine silicon thin film with F = 0 sccm shows a dominant peak at ∼620 cm−1 due to the wagging mode of Si–H vibration, whereas the extended tail towards higher wavenumber at ∼670 cm−1 corresponds to the Si–H rocking mode. With the introductory inclusion of CH4 in the plasma at F = 2 sccm, the intensity of the wagging mode has been enhanced with the appearance of a separate peak at ∼880 cm−1, which is due to the wagging and rocking modes of (SiH2)n.38,39 For F > 2 sccm, the additional peak that starts prominently appearing in the spectra at ∼780 cm−1 corresponds to the Si–C stretching mode absorption. All the absorption peaks rapidly increase in intensity at higher flow rates of CH4, F > 20 sccm.
Each spectrum has been deconvoluted into five components as identified in Fig. 4(a) and the corresponding data for the most prominent Si–C stretching band at ∼780 cm−1 has been plotted in Fig. 4(b). The peak position has been observed to shift substantially towards higher wavenumber along with rapid widening in the corresponding FWHM for increasing F till ≤20 sccm, as a consequence of larger number of C-atoms being systematically attached to the Si-network and changing the overall structural ordering of the amorphous matrix. For F > 20 sccm, however, a significantly fast shift in the peak position identifies larger numbers of C-atoms being attached to single Si-atoms. Increasing numbers of C-atom leads to elevated polarity in the Si–C bond, resulting in the shift of peak position.40 The virtual saturation of the corresponding FWHM represents a narrow spreading of bond lengths and a low distortion of the bond angles, perhaps characterizing the formation of a small fraction of the SiC crystalline state.41
The bond density of Si–C (NSi–C) has been estimated from the integrated area under the Si–C stretching mode peak using the following relation:
 | (5) |
The pristine silicon thin films have been obtained at a high deposition rate of ∼230 Å min−1, which is significantly high compared to similar films grown by commonly-used capacitively coupled plasma CVD with hydrogen dilution wherein it is typically 3 Å min−1.44 With the introduction of CH4 in the plasma, the deposition rate of the nc-Si-QD/a-SiC thin films decreases abruptly to ∼65 Å min−1 at F = 10 sccm, as shown in Fig. 5. For further addition of CH4, the deposition rate, however, virtually attains a sallow saturation. The reduced deposition rate at increased CH4 dilution arises due to the reduced SiHn radical density in the plasma. In addition, methane molecules have dissociation energy (∼413.0 kJ mol−1) higher than that of silane molecules (∼299.2 kJ mol−1) and the carbon-based radicals have a much lower sticking coefficient compared to the silicon-based radicals. These two factors act in accord and eventually lead to the lower deposition rate of the films. In addition, the reduced fraction of SiHn radicals in the plasma at higher flow rate of CH4 encounters a rather higher number of atomic H decomposed from increased total flow of feedstock (SiH4 + CH4), which further succeeds in reducing the growth rate of the material due to increased atomic H etching.45
The optical band gaps of the nc-Si-QD/a-SiC thin films have been estimated from the standard Tauc's plot of the absorption spectra in the UV-visible region following the equation given below:
 | (6) |
The electrical conductivity of the films has been studied in co-planar configuration with aluminium electrodes. The dc dark conductivity, σd, has been plotted in Fig. 7 against the CH4 flow rate. The pristine crystalline silicon thin films possess a high σd ∼ 8.2 × 10−9 S cm−1. With the incorporation of CH4 in the plasma, the conductivity has been improved significantly to 2.01 × 10−4 S cm−1 at F = 20 sccm. However, for higher F, the σd starts reducing very quickly and attains a very low magnitude of 8.47 × 10−11 S cm−1 at F = 35 sccm, wherein the film is dominantly amorphous in nature.
 | ||
| Fig. 7 The variation in the dark electrical conductivity showing very high magnitude of ∼10−4 S cm−1 at F = 20 sccm. | ||
4. Discussions
Let us now interpret the experimental results on the structural, optical and electrical properties of the nc-Si-QD/a-SiC films, as the effect of systematic inclusion of C into the highly crystalline pristine silicon network by introducing CH4 in the SiH4 plasma without any hydrogen dilution. It has been observed that the way most of the properties depend on the flow rate of CH4 (F) has two different regions. For 0 ≤ F (sccm) ≤ 20, the deposition rate decreases sharply, the carbon incorporation into the amorphous matrix is low, the overall crystallinity has been improved with minor decrease in the average size of the Si-QDs having preferred growth orientation along 〈220〉 lattice planes of silicon, finite widening in the optical band gap, although a significant improvement in the dark electrical conductivity by about four orders of magnitude. Further increase in F beyond 20 sccm lead to a sallow saturation of the deposition rate, higher Si–C bond density, sharp reduction in crystallinity with significant miniaturization of Si-QDs having grossly reduced intensity of 〈220〉 c-Si planes, a significant enhancement in the optical band gap along with a sharp lowering of the electrical conductivity by several orders of magnitude. At very high magnitude of F = 35 sccm, however, the film becomes structurally amorphous-dominated.In a multi-phase network wherein nc-Si-QDs are dispersed in the a-SiC matrix, the optical gap, Eg, is governed by the contributions from both the phases. Accordingly, Eg might have dominant contributions from two parameters: (i) average size of Si-QD along with the overall crystallinity and (ii) Si–C bond concentration in the amorphous matrix. The optical band gap, Eg, of the mixed phase material can be empirically presented as: Eg = ESi-QD + Ea-SiC, where ESi-QD arises from the quantum confinement effect of the Si-QDs and is inversely proportional to the square of their radius, whereas the Ea-SiC depends on Si–C bond density and the bonded H-content in the network.13,41,46 During the enhancement of CH4 flow rate in the range F ≤ 20 sccm, replacement of the weaker Si–Si bonds by stronger Si–C bonds helps to increase the Ea-SiC component;44 in addition, minor reduction in the sizes of Si-QDs leads to enhanced quantum confinement effects and therefore a subsequent widening of the optical gap. In the higher range of CH4 flow, F ≥ 25 sccm, in addition to a larger amount of C inclusion into the Si-network and its subsequent influences, a reasonably large reduction in the size of Si-QDs (from 4.1 to 3.1 nm) within the Bohr-radius zone induces significant band gap widening resulting in a notable overall enhancement in the Eg of the nc-Si-QD/a-SiC network, as demonstrated in Fig. 6.
It has been observed with interest that during increasing C incorporation into the Si network in the form of Si–C bonds and the subsequent widening of the optical gap of the nc-Si-QD/a-SiC network, corresponding to increasing F in the range 0 ≤ F (sccm) ≤ 20, the dark electrical conductivity increases by four orders of magnitude. From structural and elemental analysis, the nc-Si-QD/a-SiC films can be considered as an array of crystalline Si-QDs embedded in an insulating amorphous Si:H and/or SiC matrix. In such multi-phase thin film structures, the prevalent transport mechanism is based on percolation of carriers (which are thermally activated to the conduction band) between the crystalline grains through the amorphous phase.47 There are two competing factors in controlling the electrical transport properties in the films – the crystalline part with higher mobility promoting the transport properties and the insulating barrier layer of the amorphous part, controlled by the Si–C bond density, hindering the transport of charge carriers. At F = 0 sccm, the film is highly crystalline (XC = ∼76.9%) with grains of average size ∼4.5 nm, which provide a good conducting path for the carriers. In the region 0 ≤ F (sccm) ≤ 20, the crystalline volume fraction is increased as well as the size of the Si-QDs decreases. The influence of increasing number density of the crystallites with their reduced size wins over the Si–C bond density in terms of electrical transport by reducing the average inter-QD distance, resulting in the overall improvement in the dark electrical conductivity.29 In addition, the very remarkable feature of the Si-QDs is their dominant growth along the thermodynamically most favoured 〈220〉 crystallographic orientation, compared to the regular growth along the 〈111〉 direction, which reaches a magnitude as high as I〈220〉/I〈111〉 ≥ 2. The closer lattice planes of the 〈220〉 orientation provide a superior conduction path to the charge carriers compared to the 〈111〉 planes. Altogether, a significant increase in the electrical conductivity by four orders of magnitude to σd ∼ 2 × 10−4 S cm−1 has been realized. For F > 20 sccm, both the sharply reduced crystallinity and the enhanced Si–C bond density influence unidirectionally in deteriorating the electrical transport, leading to a sharp decay of the dark conductivity. At F = 35 sccm, the films become predominantly amorphous in nature with a very high density of Si–C bonds, which restrict the conductivity to a very low magnitude of 8.47 × 10−11 S cm−1.
It is noteworthy to observe that the way carbon inclusion has changed the structural, optical and electrical properties of the pristine silicon network is surprisingly unusual compared to the regular reports from available literature; however, significantly useful for device application. In general, the thin films of nano-crystalline silicon quantum dots (nc-Si-QD) embedded in amorphous silicon carbide matrix have been fabricated from the hydrogen diluted (SiH4 + CH4)-plasma in a commonly-used capacitively coupled plasma CVD. Systematic inclusion of C into the Si-network, in general, introduces deviation from crystallinity and reduction in electrical conductivity, although the optical band gap widens. On the contrary, present experimental results lead to the finite increase in crystallinity with simultaneous significant improvement in electrical conductivity while widening the optical band gap due to limited inclusion of C into the Si-network. In addition, H2-dilution not being essential in the present experiment with ICP-CVD, the nc-Si-QD/a-SiC films are obtained with a growth rate much higher than the same from conventional capacitively coupled PECVD wherein a very high degree of H2-dilution is a prerequisite in promoting crystallization to the Si-network.
In comparison with the conventional capacitively coupled plasma sources, the ICP-CVD operating in the electromagnetic mode has several major advantages, e.g., it has: (i) very high densities of the plasma species, in particular, the electron number density ∼1012 cm−3 at 10 mTorr, (ii) low plasma sheath potentials (several or tens of volts) near the deposition substrate, which is advantageous for the reduction in ion bombardment on the deposited thin films, (iii) low electron temperatures (a few electron volts) under a broad range of discharge conditions, and (iv) excellent uniformity of the plasma parameters in the radial and axial directions. High density of plasma species, e.g., SiHn/CHn (n ≤ 3) as well as atomic-H contribute for enhancing growth rate and promoting crystallization simultaneously.20,45,48 The best reported value of the deposition rate for Si-QDs in a-SiC matrix fabricated in ICP-plasma has been attained at an exorbitantly high RF power of 2000 W, which is not compatible for device fabrication.44 The present study demonstrates the growth of nc-Si-QD/a-SiC thin films prepared at a low RF power of 350 W and with a reasonably high deposition rate of 24 nm min−1, which is much higher than the typical deposition rate of 0.3 nm min−1 for similar films prepared in capacitively coupled RF-PECVD.
It was previously reported that growth along the thermodynamically preferred 〈220〉 planes is formed at elevated temperature and high pressure.49 Under particular parametric conditions of plasma, near the amorphous to nano-crystalline phase transition region, a higher 〈220〉 peak intensity can be detected compared to that for highly crystalline Si. On the contrary, the present investigation identifies the predominant growth of Si nano-crystals along the 〈220〉 planes with intensity ratio I〈220〉/I〈111〉 ≥ 2 within a highly crystalline network (∼84%) at a low pressure and moderately low temperature. Such dominant crystalline growth along the thermodynamically preferred orientation might be attributed to the inductively coupled plasma with its specific characteristics mentioned above,27,30 including its associated high density of atomic hydrogen arising from the dissociation of the SiH4 and CH4. The highly energetic atomic hydrogen helps in promoting the thermodynamically preferred growth by means of chemical annealing on the film's growing surface. The 〈220〉 oriented growth in Si nano-crystals directly stimulates the performance of solar cells. The charge carriers, whose transport path is normal to the substrate in the nc-Si solar cells in superstrate configuration, observe fewer grain boundaries in (220) oriented grains and thereby, reduced bulk recombination and/or field losses increase the open circuit voltage (VOC) and the Fill Factor (FF).50
In view of its effective application as the window layer in all silicon p–i–n solar cells in superstrate configuration, the nc-Si-QD/a-SiC films must have wide optical gap with very high electrical conductivity. However, generally, a trade-off relation holds between these two optical and electrical properties. The present investigation identifies some deviation in this perpetual trade-off relation; the dark conductivity has been improved significantly even with the widening of the optical gap in nc-Si-QD/a-SiC films during incorporation of a small amount of C in a stringent parametric condition within ICP-CVD. In this context, a comparative study has been made on the optical gap vs. dark conductivity relation among various undoped a-/μc-/nc-silicon–carbon-alloy thin films available in the literature. As shown in Fig. 8, the studies by Yunaz et al., Demichelis et al. and Li et al. have shown very poor conductivity for amorphous silicon carbide films compared to the present data at equivalent band gaps.51–53 In the report by Lee et al., the nc-Si/a-SiC thin films showed very high optical gap with conductivity comparatively higher than the a-SiC films.12 However, the band gap was reported in terms of E04, which is supposed to be higher than the Tauc's optical gap discussed here. Among the referenced sets of data, only those from Coscia et al. and the present report have closer conductivity at around Eg ∼ 1.90 eV; however, the conductivity for the present set of samples is superior for the region of band gap 1.9 ≤ Eg (eV) ≤ 2.04.10 However, there are several reports that demonstrate the development of μc-SiC thin films having very high electrical conductivity even at a wider optical band gap.54,55 Such thin films have been utilized by Hamakawa et al. as the window layer in the hetero-junction solar cells with a-Si:H i-layer in the glass/TCO/p-μc-SiC/i-a-Si:H/n-μc-Si/Ag configuration.56 However, in view of developing nc-Si solar cells, the spontaneous subsequent growth of the nc-Si i-layer on the μc-SiC thin films will be obstructed due to the lattice mismatch between crystalline SiC and Si. To surpass such an obstacle, another layer needs to be deposited in between those two layers,57 which leads to clear technological inconvenience. On the contrary, the present nc-Si-QD/a-SiC thin films, developed at a reasonably high growth rate from spontaneous (SiH4 + CH4)-plasma processing without H2-dilution by ICP-CVD, with its wide optical band gap and high electrical conductivity, possess adequate prospects for their future use in the window layer of nc-Si solar cells, in particular.
5. Conclusions
The present manuscript deals with the synthesis of nanocrystalline Si quantum dots embedded in an amorphous SiC matrix (nc-Si-QD/a-SiC) with a high deposition rate from the gas mixture of silane and methane without any additional hydrogen dilution, using spontaneous plasma processing by inductively coupled plasma CVD. The CH4 flow in the plasma acts as dual source of both carbon and atomic hydrogen. During limited increase in CH4 concentration in the plasma, increasing C-incorporation into the amorphous matrix surrounding the crystalline silicon quantum dots (Si-QDs) continuously miniaturizes the size of the Si-QDs. However, simultaneous increase in atomic-H extracted from CH4 plays over the effect of C-inclusion in introducing newer nucleation sites and thereby enhances the overall crystallinity. In addition, atomic H at higher density promotes the Si crystallization along the thermodynamically favourable 〈220〉 crystallographic orientation, compared to 〈111〉, by means of chemical annealing. The optical gap widens due to the larger number of Si–C bonds in the amorphous network and the increased quantum confinement effect on the miniaturized Si-QDs at increasing CH4 concentration in the SiH4 plasma. The presence of Si-QDs with a preferred 〈220〉 crystallographic orientation available in the hetero-structure at the higher crystalline volume fraction contribute simultaneously in maintaining high electrical conductivity in the nc-Si solar cells in superstrate configuration. Altogether, the nc-Si-QD/a-SiC films demonstrate a significantly high electrical conductivity, ∼10−4 S cm−1, at a wide optical band gap, ∼1.90 eV, with which the conductivity for the present set of samples, in the region of band gap 1.9 ≤ Eg (eV) ≤ 2.04, has been found to be superior to the data available in the literature.Finally, the present study enables the spontaneous miniaturization of self-assembled nc-Si-QDs with preferred 〈220〉 crystallographic orientation and a rapid growth of superior quality nc-Si-QD/a-SiC thin films of enhanced crystallinity, maintaining a combination of wide optical gap and simultaneous high electrical conductivity; after imminent doping, these could be efficiently utilized as window layers in third generation all-silicon solar cells.
Acknowledgements
The study has been carried out under nano-silicon projects funded by the Department of Science and Technology (Nano-Mission Program) and the Council of Scientific and Industrial Research, Government of India. The HR-TEM studies have been performed using facilities of the Unit on Nano-Science at IACS.References
- Y. Tawada, M. Kondo, H. Okamoto and Y. Hamakawa, Sol. Energy Mater., 1982, 6, 299–315 CrossRef CAS.
- Y. Matsumoto, F. Meléndez and R. Asomoza, Sol. Energy Mater. Sol. Cells, 2001, 66, 163–170 CrossRef CAS.
- D. Das, M. Jana and A. K. Barua, Sol. Energy Mater. Sol. Cells, 2000, 63, 285–297 CrossRef CAS.
- D. Das and A. K. Barua, Sol. Energ. Mater. Sol. Cells, 2000, 60, 167–179 CrossRef CAS.
- S. Y. Myong, S. S. Kim and K. S. Lim, J. Appl. Phys., 2004, 95, 1525–1530 CrossRef CAS.
- D. Das and A. Samanta, Nanotechnology, 2011, 22, 055601 CrossRef PubMed.
- P. D. Veneri, L. V. Mercaldo and I. Usatii, Appl. Phys. Lett., 2010, 97, 023512 CrossRef.
- S. Ogawa, M. Okabe, T. Itoh, N. Yoshida and S. Nonomura, Thin Solid Films, 2008, 516, 758–760 CrossRef CAS.
- B. Sain and D. Das, Phys. Chem. Chem. Phys., 2013, 15, 3881–3888 RSC.
- U. Coscia, G. Ambrosone, S. Lettieri, P. Maddalena, P. Rava and C. Minarini, Thin Solid Films, 2003, 427, 284–288 CrossRef CAS.
- U. Coscia, G. Ambrosone, S. Lettieri, P. Maddalena, V. Rigato, S. Restello, E. Bobeico and M. Tucci, Sol. Energ. Mater. Sol. Cells, 2005, 87, 433–444 CrossRef CAS.
- J. E. Lee, S. K. Ahn, J. H. Park, J. Yoo, K. H. Yoon, D. Kim and J. S. Cho, Prog. Photovolt: Res. Appl., 2015, 23, 1715–1723 CrossRef CAS.
- D. Kar and D. Das, J. Mater. Chem. A, 2013, 1, 14744–14753 CAS.
- Z. Hu, X. Liao, H. Diao, Y. Cai, S. Zhang, E. Fortunato and R. Martins, J. Non-Cryst. Solids, 2006, 352, 1900–1903 CrossRef CAS.
- T. Fujibayashi and M. Kondo, J. Appl. Phys., 2006, 99, 043703 CrossRef.
- K. Adhikary and S. Ray, J. Non-Cryst. Solids, 2007, 353, 2289–2294 CrossRef CAS.
- D. Das and M. Jana, Sol. Energ. Mater. Sol. Cells, 2004, 81, 169–181 CrossRef CAS.
- H. Jia, H. Shirai and M. Kondo, J. Appl. Phys., 2007, 101, 114912 CrossRef.
- D. Raha and D. Das, Sol. Energ. Mater. Sol. Cells, 2011, 95, 3181–3188 CrossRef CAS.
- M. A. L. a. A. J. Lichtenberg, Principles of PlasmaDischarges and Materials Processing, Wiley Interscience, New York, 1994 Search PubMed.
- Y. Wei, L. Wanbing, H. Li and F. Guangsheng, J. Phys. D: Appl. Phys., 2004, 37, 3304 CrossRef.
- K. N. Ostrikov, S. Xu and M. Y. Yu, J. Appl. Phys., 2000, 88, 2268–2271 CrossRef CAS.
- D. Das and D. Kar, Phys. Chem. Chem. Phys., 2014, 16, 25421–25431 RSC.
- H. K. John, Plasma Sources Sci. Technol., 1996, 5, 166 CrossRef.
- C. Qijin, S. Xu, J. D. Long, Z. H. Ni, A. E. Rider and K. Ostrikov, J. Phys. D: Appl. Phys., 2008, 41, 055406 CrossRef.
- S. Xu, K. N. Ostrikov, Y. Li, E. L. Tsakadze and I. R. Jones, Phys. Plasmas, 2001, 8, 2549–2557 CrossRef CAS.
- D. Raha and D. Das, Appl. Surf. Sci., 2013, 276, 249–257 CrossRef CAS.
- C. H. Cheng, Y. H. Lin, J. H. Chang, C. I. Wu and G. R. Lin, RSC Adv., 2012, 4, 18397 RSC.
- D. Das and B. Sain, J. Appl. Phys., 2013, 114, 073708 CrossRef.
- B. Sain and D. Das, Sci. Adv. Mater., 2013, 5, 188–198 CrossRef CAS.
- S. Guha, G. Hendershot, D. Peebles, P. Steiner, F. Kozlowski and W. Lang, Appl. Phys. Lett., 1994, 64, 613–615 CrossRef.
- Y. L. He, Y. Y. Wei, G. Z. Zheng, M. B. Yu and M. Liu, J. Appl. Phys., 1997, 82, 3408–3413 CrossRef CAS.
- G. Z. Yue, J. D. Lorentzen, J. Lin, D. X. Han and Q. Wang, Appl. Phys. Lett., 1999, 75, 492–494 CrossRef CAS.
- M. Jana, D. Das and A. K. Barua, Sol. Energ. Mater. Sol. Cells, 2002, 74, 407–413 CrossRef CAS.
- V. Paillard, P. Puech, M. A. Laguna, R. Carles, B. Kohn and F. Huisken, J. Appl. Phys., 1999, 86, 1921–1924 CrossRef CAS.
- Q. Cheng, E. Tam, S. Xu and K. Ostrikov, Nanoscale, 2010, 2, 594–600 RSC.
- D. Das, Thin Solid Films, 2005, 476, 237–245 CrossRef CAS.
- G. Lucovsky, J. Yang, S. S. Chao, J. E. Tyler and W. Czubatyj, Phys. Rev. B, 1983, 28, 3225–3233 CrossRef CAS.
- A. Samanta and D. Das, Sol. Energ. Mater. Sol. Cells, 2009, 93, 588–596 CrossRef CAS.
- U. Coscia, G. Ambrosone and D. K. Basa, J. Appl. Phys., 2008, 103, 063507 CrossRef.
- T. Rajagopalan, X. Wang, B. Lahlouh, C. Ramkumar, P. Dutta and S. Gangopadhyay, J. Appl. Phys., 2003, 94, 5252–5260 CrossRef CAS.
- D. K. Basa and F. W. Smith, Thin Solid Films, 1990, 192, 121–133 CrossRef CAS.
- C. Summonte, R. Rizzoli, M. Bianconi, A. Desalvo, D. Iencinella and F. Giorgis, J. Appl. Phys., 2004, 96, 3987–3997 CrossRef CAS.
- Q. Cheng, S. Xu and K. Ostrikov, Acta Mater., 2010, 58, 560–569 CrossRef CAS.
- D. Das, Solid State Phenom., 1995, 44, 227 CrossRef.
- N.-M. Park, C.-J. Choi, T.-Y. Seong and S.-J. Park, Phys. Rev. Lett., 2001, 86, 1355–1357 CrossRef CAS PubMed.
- R. Robertson and A. Gallagher, J. Chem. Phys., 1986, 85, 3623–3630 CrossRef CAS.
- Q. Cheng, S. Xu and K. Ostrikov, J. Phys. Chem. C, 2009, 113, 14759–14764 CAS.
- V. L. Dalal, K. Muthukrishnan, X. Niu and D. Stieler, J. Non-Cryst. Solids, 2006, 352, 892–895 CrossRef CAS.
- B. E. Vallat-Sauvain, Advances in Microcrystalline Silicon Solar Cell Technologies in Thin Film Solar Cells, in Fabrication, Characterization, and Application, ed. J. P. a. V. Arkhipov, John Wiley and Sons, England, 2007 Search PubMed.
- S. X. Li, Y. Q. Cao, J. Xu, Y. J. Rui, W. Li and K. J. Chen, Appl. Surf. Sci., 2013, 270, 287–291 CrossRef CAS.
- I. A. Yunaz, K. Hashizume, S. Miyajima, A. Yamada and M. Konagai, Sol. Energ. Mater. Sol. Cells, 2009, 93, 1056–1061 CrossRef CAS.
- F. Demichelis, G. Crovini, C. F. Pirri, E. Tresso, G. Amato, U. Coscia, G. Ambrosone and P. Rava, Thin Solid Films, 1994, 241, 274–277 CrossRef CAS.
- T. Chen, Y. Huang, D. Yang, R. Carius and F. Finger, Thin Solid Films, 2011, 519, 4523–4526 CrossRef CAS.
- S. Miyajima, J. Irikawa, A. Yamada and M. Konagai, Appl. Phys. Lett., 2010, 97, 023504 CrossRef.
- Y. Hattori, D. Kruangam, T. Toyama, H. Okamoto and Y. Hamakawa, Appl. Surf. Sci., 1988, 33–34, 1276–1284 CrossRef.
- Y. Huang, T. Chen, A. Gordijn, A. Dasgupta, F. Finger and R. Carius, J. Non-Cryst. Solids, 2008, 354, 2430–2434 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |







