Catalytic wet oxidation of phenol with Fe–ZSM-5 catalysts
Ying Yan,
Songshan Jiang and
Huiping Zhang*
School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510640, PR China. E-mail: hpzhang@scut.edu.cn; Fax: +86 2087111975; Tel: +86 2087111975
First published on 3rd December 2015
Abstract
Fe–ZSM-5 and Fe2O3/ZSM-5 zeolite catalysts were prepared and tested for catalytic wet oxidation of phenol. First, Fe–ZSM-5 and Fe2O3/ZSM-5 zeolite catalysts were prepared by the hydrothermal synthetic and incipient wetness impregnation method and characterized to determine the framework and extra-framework Fe3+ species. Second, the catalytic properties of Fe–ZSM-5 in the oxidation of phenol were systematically studied to determine the optimum technological parameters by investigating the effects of reaction temperature, pH, catalyst concentration and stirring rate on the conversion of phenol. In addition to the phenol conversion, selectivity to CO2 and concentration of aromatic intermediates in the oxidation of phenol with the two catalysts were analyzed under the same optimum conditions. Leaching of iron from the catalysts, as well as the catalytic stability of Fe–ZSM-5, was also tested. Finally, the kinetics of catalytic wet oxidation of phenol with Fe–ZSM-5 was studied. The experimental results showed that both the framework and extra-framework Fe3+ species were present in Fe–ZSM-5. The oxidation reaction with Fe–ZSM-5 was performed well at a temperature of 70 °C, pH of 4, catalyst concentration of 2.5 g L−1, stirring rate of 400 rpm and reaction time of 180 min. The conversion of phenol reached 94.1%. From the catalytic results of the two catalysts, it can be concluded that the framework Fe3+ species may be more efficient in phenol oxidation than the extra-framework Fe3+ species, the stability of Fe–ZSM-5 was better and a relatively low decrease in activity could be found after three consecutive runs. The activation energy of 27.42 kJ mol−1 was obtained for phenol oxidation with Fe–ZSM-5.
1. Introduction
Phenol is a typical pollutant from industrial processes that appears especially in wastewater from refineries, and petrochemical plants, pharmaceutical plants.1,2 Moreover, how to efficiently eliminate phenol from water and wastewater is still a focus of many researchers.Various techniques, such as biological, physical and chemical treatments, have been used to purify the abovementioned industrial organic wastewaters.3–5 However, the conventional biological method is not widely used to treat highly non-biodegradable wastewater and also requires considerably long residence time for micro-organisms to degrade the pollutants.6 Physical treatments, for example, adsorptive processes, which are useful in the purification of diluted wastewaters, need a further destructive post-treatment.7 Advanced oxidation processes are much more effective in decomposing a wide range of organic pollutants, especially for high concentration phenolic wastewater (>1000 mg L−1), among the chemical treatment technologies.8 There are a lot of different available oxidants, such as ozone, oxygen, and hydrogen peroxide as well as some combinations, such as H2O2/UV or H2O2/O2, can be used in advanced oxidation processes. Catalytic wet peroxide oxidation, based on the Fenton-type process, is considered a relatively low cost and highly efficient process for organic pollutants;9 however, the use of metallic salts as catalysts is precipitated as Fe(OH)3, which results in an additional pollutant. To avoid additional pollution, heterogeneous catalysis is a good choice to minimize the concentration of transition metal ions generated during the reaction, and these heterogeneous catalysts are always in the form of active iron over supports.10 Zeolites, as the porous material, have been widely used as an effective catalyst support in the environment, in industries and in other important areas.11–13 Recent studies have demonstrated the preparation of a series of Fe-bearing solid catalysts such as iron-supported meso-structured silica supports,14,15 activated carbon impregnated with iron,16 iron modified zeolites,17,18 and other iron catalysts19 for use in catalytic wet peroxide oxidation of phenolic wastewater. Among these iron catalysts, Fe–ZSM-5 as a heterogeneous catalyst earlier used in NOx reduction20 and catalytic wet peroxide oxidation of phenol wastewater was studied synthetically and for catalytic activity,21,22 including the synthesis process, catalytic activity and deactivation. Although Fe–ZSM-5 has been studied as a catalyst in the oxidation of phenol in recent years, to the best of our knowledge, there are few works on the systematic research of catalytic wet oxidation of phenol with Fe–ZSM-5.
The purpose here is to study the catalytic properties of the framework and extra-framework Fe3+ species in two types of iron zeolite catalysts for wet oxidation of phenol with H2O2 as the oxidant. In addition, a systematic work on the characterization, technological parameters, selectivity to CO2, aromatic intermediates, stability and activation energy will be presented in this study.
2. Experimental
2.1 Materials
Phenol was purchased from the Guangzhou Chemical Reagent Factory. Hydrogen peroxide (H2O2, 30 wt% aqueous) was purchased from the Shanghai Qiangshun Chemical Reagent Factory. Tetrapropylammonium hydroxide (TPAOH) was purchased from the Tianjin Guangfu Fine Chemical Research Institute. Tetraethoxysilane (TEOS) was purchased from the Tianjin Fuchen Chemical Reagent Factory. Sodium aluminate (NaAlO2) was purchased from the Sinopharm Chemical Reagent Co., Ltd. Fe(NO3)3·9H2O was purchased from the Tianjin Damao Chemical Reagent Factory. ZSM-5 zeolites (Si/Al = 50) with an average particle diameter of 1 mm were purchased from the Nankai University Catalyst Factory. Catechol, hydroquinone, resorcinol and benzoquinone purchased from the Sinopharm Chemical Reagent Co., Ltd. were all used as standard samples for the test. Deionized water was used in all synthetic processes. All the chemical reagents used in this study were analytical grade.2.2 Preparation of Fe–ZSM-5 catalysts
Fe–ZSM-5 zeolite catalyst was synthesized by hydrothermal synthesis from a mixture with the molar ratio 5000 H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 TEOS
100 TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 TPAOH
10 TPAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 NaAlO2
2 NaAlO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Fe(NO3)3·9H2O. The mixture was aged 24 h and treated hydrothermally in a 50 mL Teflon lined autoclave at 170 °C for 48 h. The resulting white solid was filtered, ultrasonically washed by deionized water for 20 min and dried at 100 °C, then calcined at 550 °C under air for 4 h.
2 Fe(NO3)3·9H2O. The mixture was aged 24 h and treated hydrothermally in a 50 mL Teflon lined autoclave at 170 °C for 48 h. The resulting white solid was filtered, ultrasonically washed by deionized water for 20 min and dried at 100 °C, then calcined at 550 °C under air for 4 h.
Fe2O3/ZSM-5 zeolite catalyst was prepared by the incipient wetness impregnation method, involving the impregnation of ZSM-5 with the proper amount of Fe(NO3)3·9H2O. After impregnation, the catalyst was dried at 110 °C for 24 h and then calcined at 550 °C under air for 8 h. The Fe load was adjusted to 1.5 wt% in both of the prepared catalysts.
2.3 Catalyst characterization
Different techniques were used for the characterization of the catalysts. X-ray diffraction (XRD) using a D8 ADVANCE (Bruker Co.) diffractometer with Cu Kα radiation (40 kV, 40 mA) with 2θ range of 5–60° was used to determine the crystalline phases present in both the catalysts and the supports. The structure properties of the catalysts were analyzed by Fourier transform infrared (FTIR, Spectnlm2000, Perkin Elmer, USA) spectrometry with a resolution of 4 cm−1 in the range from 400 to 4000 cm−1 at room temperature. A typical pellet containing 1 wt% of sample was prepared by mixing 1 mg of sample with 100 mg KBr. Temperature programmed reduction (TPR) tests were performed on Quantachrome Automated Chemisorption Analyzer in a flowing H2 reduction system with a TCD detector. 50 mg of each sample was loaded into the reactor and purged with Ar at a rate of 50 mL min−1 at 350 °C for 30 min to eliminate contaminants and then cooled down to 50 °C. The temperature was increased to 900 °C at a heating rate of 10 °C min−1 with a flowing gas mixture of 10% H2 and 90% Ar. The structure modifications involving Fe3+ species were identified by UV-Vis reflectance spectra carried out on a UV spectrophotometer (U-3010, Hitachi, Japan) in the 200–700 nm wavelength range employing BaSO4 as the blank.2.4 Catalytic wet oxidation of phenol
The oxidation of phenol was carried out in a 250 mL stoppered glass flask in a water bath with a condenser, thermocouple and pH electrode at a certain stirring rate. A volume of 200 mL of a 2500 mg L−1 aqueous phenol solution and a certain weight of Fe–ZSM-5 zeolite catalyst were transferred into the flask. The experiments were carried out at different temperatures between 40 and 80 °C, pH = 2–6 and stirring rates between 200 and 600 rpm. The H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phenol molar ratio was fixed at 21, whereas for the complete oxidation of phenol to CO2 and H2O, the stoichiometric ratio is 14. The amount of H2O2 used was in excess, which was equal to 1.5 times of the H2O2 amount necessary to completely oxidize phenol to CO2 and H2O. When the desired reaction temperature was reached, H2O2 was dropped into the flask, and the reaction was then considered to be started. In addition, the effects of different catalyst loads (0.5–5 g L−1) were also tested in these experiments.
phenol molar ratio was fixed at 21, whereas for the complete oxidation of phenol to CO2 and H2O, the stoichiometric ratio is 14. The amount of H2O2 used was in excess, which was equal to 1.5 times of the H2O2 amount necessary to completely oxidize phenol to CO2 and H2O. When the desired reaction temperature was reached, H2O2 was dropped into the flask, and the reaction was then considered to be started. In addition, the effects of different catalyst loads (0.5–5 g L−1) were also tested in these experiments.
Liquid samples were taken at different time intervals and immediately analyzed. The concentration of phenol and the identification of intermediates were carried out in a HPLC (model 1100, Agilent Technologies, USA) with a HC-C18 reverse phase column (5 μm × 250 mm × 4.6 mm). The total organic carbon (TOC) values were obtained by a TOC Analyzer (Sievers Innov, General Electric, USA).
The calculation method given by Nguyen et al.23 was applied to determine the conversion of phenol (Xphenol, %) and selectivity to carbon dioxide (SCO2, %).
2.5 Stability tests
Leaching tests were carried out to investigate whether small amounts of the dissolved iron were responsible for the observed catalytic activity. The ion contents of the reaction solution were measured at different time intervals by atomic adsorption spectroscopy (AA240FS, Varian, USA).Three consecutive runs were conducted with the same Fe–ZSM-5 zeolite catalyst load at 70 °C and pH of 4 after simply drying at 100 °C. The conversion of phenol was tested at different time intervals to analyze the stability of the zeolite catalyst.
3. Results and discussion
3.1 Characterization of catalysts
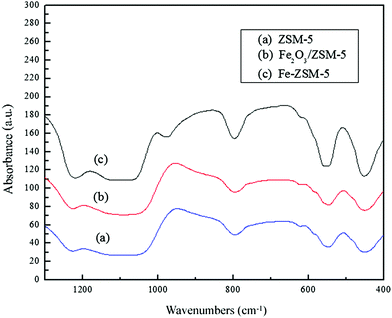
To confirm the structure and the crystallinity, different samples were analyzed by using XRD. The XRD patterns of ZSM-5, Fe2O3/ZSM-5 and Fe–ZSM-5 are shown in Fig. 1. In the XRD patterns, all the samples give the same diffraction peaks in the ranges of 2θ = 7–9° and 2θ = 23–25°, which match well with the reports on MFI-structure.24 It is known that metal-impregnation affects the structure regularity of zeolites as found by other researchers.17,25,26 As we can observe in the XRD patterns of Fe–ZSM-5, the higher intensities of diffraction peaks appear at 2θ = 7–9° compared with the other two samples, which suggests that Fe3+ may be successfully incorporated into the MFI framework.27 In addition, in the XRD patterns of Fe–ZSM-5 and Fe2O3/ZSM-5, there are two weak diffraction peaks for α-Fe2O3 at 2θ = 33° and 36°, which infers the presence of isolated extra-framework Fe3+ species in both Fe–ZSM-5 and Fe2O3/ZSM-5. The intensity decrease of the XRD patterns at 2θ = 23–25° of the Fe–ZSM-5 and Fe2O3/ZSM-5 may also confirm the existence of Fe species.Infrared spectroscopy is a tool widely used to characterize the structural properties of zeolites. Useful information can be obtained by exploring the framework (1350–400 cm−1) regions.28 The IR spectra of ZSM-5, Fe2O3/ZSM-5 and Fe–ZSM-5 are shown in Fig. 2. IR spectra of all the samples show that the same absorption peaks centered at 1225 cm−1, 1093 cm−1, 790 cm−1, 550 cm−1 and 450 cm−1, are present and match with the skeletal vibrations of the MFI zeolite structure (ZSM-5). The bands at 550 cm−1 are assigned to the vibration of double 5-rings in the MFI lattice and the ratio of band intensities at 500 and 450 cm−1 is often used as an indication of zeolite crystallinity.23 From the IR spectra of Fe–ZSM-5, there is an absorption peak centered at 980 cm−1. The absorption peak centered at 960 cm−1 is considered as the vibrations of SiOδ−⋯Txδ+, where Txδ+ is the heteroatom located in the framework.29 In some reports,30 the bands around 1000 cm−1 can be explained by attributing it to the local structure surrounding the framework Fe3+ species. It is described by 4 [O3Si–O]− units, which can be also used to support the framework Fe3+ species present in Fe–ZSM-5.
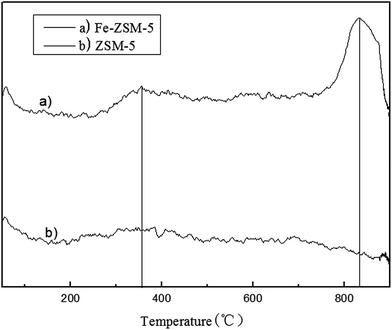
Temperature-programmed reduction (TPR) experiments were carried out to further study the iron species and their redox properties.30,31 The TPR curves of ZSM-5 and Fe–ZSM-5 are shown in Fig. 3. The TPR curve of Fe–ZSM-5 clearly shows a maximum centered at 843 °C. The peak observed at this temperature can be ascribed to a residual fraction of the framework Fe3+ species with difficult reducibility, because the reduction of framework Fe3+ species usually occurs at a temperature higher than 677 °C.32 Moreover, as we can observe in the TPR curve of Fe–ZSM-5, there is a weak signal peak at 353 °C, compared with the TPR curve of ZSM-5. It has been proposed that the iron, mostly in the form of Fe2O3, on the surface of Fe–ZSM-5 is reduced from Fe3+ to Fe2+ at around 400 °C.33
UV-Vis spectroscopy is applied to analyze the structural modifications involving the Fe3+ species.30 The UV-Vis reflectance spectrum of Fe–ZSM-5 is shown in Fig. 4. Two strong absorption peaks appeared between 200 nm and 300 nm with ligand to metal Fe3+ charge transfer character involving isolated framework Fe3+. Moreover, the appearance of a weak broad absorption was centered at 380 nm. It can be ascribed to the Fe3+ species present in the form of Fe2O3 particles.
From these results, it could be concluded that both the framework and extra-framework Fe3+ species are both present in the Fe–ZSM-5, but only the extra-framework Fe3+ species in the Fe2O3/ZSM-5 was prepared by incipient wetness impregnation.
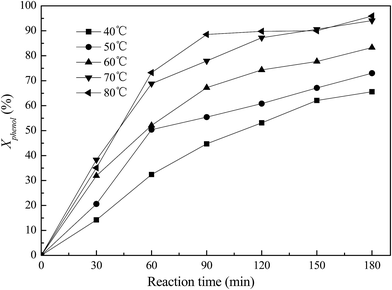
3.2 Phenol oxidation with Fe–ZSM-5
3.3 Catalyst activity with framework Fe
To investigate the contribution of framework and extra-framework Fe3+ species in the oxidation of phenol, the conversion of phenol and selectivity to CO2 with Fe–ZSM-5 and Fe2O3/ZSM-5 (1.5 wt% Fe) under the same operating conditions (pH = 4, T = 70 °C and stirring rate = 400 rpm) were analyzed and the results are shown in Fig. 9 and 10, respectively. It can be observed in Fig. 9 that the conversion of phenol over Fe2O3/ZSM-5 reaches 100%, which is clearly higher than that over Fe–ZSM-5. In other words, the activity of extra-framework Fe3+ species is higher than that of the framework Fe3+ species because the extra-framework Fe3+ species, present in the form of iron oxide (Fe2O3), are located in the pores as well as on the surface of the zeolites, which indicates higher activity. However, as can be observed in Fig. 10, Fe–ZSM-5 shows a better selectivity to CO2 (90.6%) compared with Fe2O3/ZSM-5 (only 77.6%), which indicates that framework Fe3+ species can more efficiently catalyze the destruction of the phenol ring and convert it into CO2 than extra-framework Fe3+ species.23 Two forms of Fe3+ species existed in the Fe–ZSM-5, namely, extra-framework and framework Fe3+ species, but only the extra-framework Fe3+ species existed in the Fe2O3/ZSM-5. The extra-framework Fe3+ species may have higher catalytic activity but lower stability. The higher activity of extra-framework Fe3+ species will decompose the H2O2 into O2 directly, which resulted in lower CO2 selectivity. However, the Fe–ZSM-5 catalyst can produce more ·OH radicals by catalyzing H2O2 with higher utilization efficiencies that resulted in higher CO2 selectivity when compared with Fe2O3/ZSM-5. | ||
Fig. 9 Conversion of phenol with Fe–ZSM-5 and Fe2O3/ZSM-5 ([phenol]0: 2500 mg L−1, [H2O2]0: 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1, catalyst concentration = 2.5 g L−1, pH = 4, T = 70 °C, and stirring rate = 400 rpm). 000 mg L−1, catalyst concentration = 2.5 g L−1, pH = 4, T = 70 °C, and stirring rate = 400 rpm). | ||
 | ||
Fig. 10 Selectivity to CO2 with Fe–ZSM-5 and Fe2O3/ZSM-5 ([phenol]0: 2500 mg L−1, [H2O2]0: 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1, catalyst concentration = 2.5 g L−1, pH = 4, T = 70 °C, and stirring rate = 400 rpm). 000 mg L−1, catalyst concentration = 2.5 g L−1, pH = 4, T = 70 °C, and stirring rate = 400 rpm). | ||
To learn more about the potential feasibility of framework and extra-framework Fe3+ species in the oxidation of phenol, aromatic intermediate concentrations in the oxidation with Fe–ZSM-5 and Fe2O3/ZSM-5 were tested to evaluate the ecotoxicity during this oxidation process. HPLC was used to identify the aromatic intermediates, and the distribution curves of identified aromatic intermediates are shown in Fig. 11. It is known that the most likely pathway for oxidative destruction of phenol includes the primary products corresponding to aromatics resulting from phenol hydroxylation, followed by evolution to simple carboxylic acids and finally completing oxidation to CO2 and H2O. From Fig. 11, four aromatic intermediates as well as the TOC values were tested. It was found that lower aromatic intermediate concentrations were generated in the oxidation of phenol over Fe–ZSM-5 compared with that over Fe2O3/ZSM-5, which suggests relatively lower values of ecotoxicity in the oxidation of phenol over Fe–ZSM-5. However, the reaction time also plays a very important role in the oxidation, and it must reach sufficiently low concentrations of aromatic intermediates that are much more toxic than phenol itself.40 In addition, the TOC reduction over these two zeolites catalysts after 180 min both came to at least 80%. The residual TOC is a contribution from the simple carboxylic acids, which are more resistant to oxidation, evolved by the primary products from phenol ring opening and oxidation of the aromatic intermediates.41
3.4 Stability and reusability of the catalysts
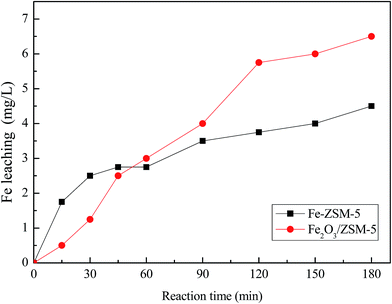
Leaching tests were also carried out over these two catalysts under the same reaction conditions to study much more about the stability of the catalysts in the oxidation process, and the results are shown in Fig. 12. It is observed that iron loading in the Fe–ZSM-5 and Fe2O3/ZSM-5 after the leaching tests were 1.32 wt% and 1.24 wt%, respectively. Insignificant reduction in iron loading during the reaction indicates that the two catalysts are not prone to leaching, compared with the iron loading of 1.5 wt% in fresh catalyst. However, a remarkable decrease of iron loading in the Fe2O3/ZSM-5 catalyst was still found when compared with the Fe–ZSM-5 catalyst.From the results abovementioned, the reusability of Fe–ZSM-5 was carried out over three consecutive runs under the optimum reaction conditions, and the results are shown in Fig. 13. The conversion of phenol decreases from 94.1% at the 1st run to 85.6% at the 3rd run. Many reports10,42,43 concluded that the decrease of catalyst activity cannot be attributed to Fe leaching from the catalyst. One important fact is the presence of residual carbon-containing matter over the surface of the used catalysts due to the absorption of residual organic compounds onto the catalyst. Many methods, such as washing by solvent and calcining at high temperature, are used to recover the catalyst and thus, more experiments will be performed in the future for a better understanding of catalyst regeneration.
3.5 Kinetics of catalytic wet oxidation of phenol with Fe–ZSM-5
A plot of initial rate of phenol (rA0) vs. stirring rate was drawn in the range of 200–600 rpm for the external diffusion elimination; moreover, a plot of initial rate (rA0) vs. particle size of Fe–ZSM-5 zeolite catalyst was also drawn in the range of 20–120 mesh for the internal diffusion elimination in this kinetic experiment under the optimum experimental conditions, and the results are shown in Fig. 14 and 15. It is easily found that the external diffusion and internal diffusion are eliminated at the stirring rate of 400 rpm and particle size of 80 meshes.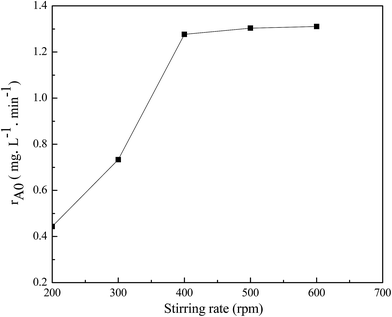 | ||
Fig. 14 Effect of stirring rate on the initial rate of phenol conversion ([phenol]0: 2500 mg L−1, [H2O2]0: 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1, catalyst concentration = 2.5 g L−1, pH = 4, and T = 70 °C). 000 mg L−1, catalyst concentration = 2.5 g L−1, pH = 4, and T = 70 °C). | ||
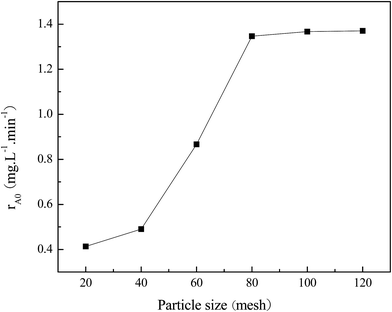 | ||
Fig. 15 Effect of catalyst particle size on the initial rate of phenol conversion ([phenol]0: 2500 mg L−1, [H2O2]0: 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1, catalyst concentration = 2.5 g L−1, pH = 4, and T = 70 °C). 000 mg L−1, catalyst concentration = 2.5 g L−1, pH = 4, and T = 70 °C). | ||
The catalytic wet oxidation of phenol over Fe–ZSM-5 with excess H2O2 can be assumed to be a first-order reaction, and the reaction rate equation is:
 | (1) |
The oxidation rates were tested for first-order kinetics by plotting ln(CA0/CA) vs. time at different temperatures from 40 °C to 70 °C, as shown in Fig. 16. The slopes of the straight lines were obtained and the values of the first-order rate coefficient are showed in Table 1. These results confirm that the catalytic reactions usually follow first-order kinetics as in related investigation.25,44
| T/K | k × 10−3 (min−1) | R2 |
|---|---|---|
| 313 | 6.2 | 0.9931 |
| 323 | 7.0 | 0.9548 |
| 333 | 9.8 | 0.9809 |
| 343 | 15.6 | 0.9917 |
Fig. 17 presents the Arrhenius plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k vs. 1/T obtained at different temperatures from 40 °C to 70 °C. From the slope of the Arrhenius plot in Fig. 17, the activation energy was calculated to be 27.42 kJ mol−1. Consequently, the initial oxidation rate can be expressed by the following equation:
k vs. 1/T obtained at different temperatures from 40 °C to 70 °C. From the slope of the Arrhenius plot in Fig. 17, the activation energy was calculated to be 27.42 kJ mol−1. Consequently, the initial oxidation rate can be expressed by the following equation:
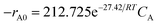 | (2) |
Similar results for activation energy of wastewaters by catalytic wet oxidation have been reported. For example, Xu et al.45 calculated an activation energy of 25.21 kJ mol−1 in the homogeneous catalytic Fenton oxidation of Reactive Brilliant Blue X-BR azo dye.
4. Conclusions
Fe–ZSM-5 and Fe2O3/ZSM-5 zeolite catalysts with 1.5 wt% Fe for catalytic wet oxidation of phenol were prepared by the hydrothermal synthetic and incipient wetness impregnation methods, respectively. Both the framework and extra-framework Fe3+ species were present in Fe–ZSM-5, but only extra-framework Fe3+ species in Fe2O3/ZSM-5. The oxidation reaction with Fe–ZSM-5 performed well under atmospheric pressure, temperature of 70 °C, pH of 4, with a catalyst concentration of 2.5 g L−1, a stirring rate of 400 rpm and reaction time of 180 min. The conversion of phenol reached 94.1%. Both the framework and extra-framework Fe3+ species could catalyze the oxidation of phenol; however, the framework Fe3+ species can be more efficient at oxidizing phenol completely into CO2. The Fe–ZSM-5 zeolite catalyst showed better stability than Fe2O3/ZSM-5, and a relatively low decrease of activity after three consecutive runs. The activation energy of oxidation with Fe–ZSM-5 zeolite catalyst was calculated to be 27.42 kJ mol−1.Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 21176086 and Grant No. 21006030) for this study.References
- G. Busca, S. Berardinelli, C. Resini and L. Arrighi, J. Hazard. Mater., 2008, 160, 265–288 CrossRef CAS PubMed.
- P. Zhang, Y. Gong, H. Li, Z. Chen and Y. Wang, RSC Adv., 2013, 3, 5121–5126 RSC.
- S. Collado, A. Laca and M. Diaz, J. Environ. Manage., 2012, 102, 65–70 CrossRef CAS PubMed.
- N. Chaouati, A. Soualah and M. Chater, Cron. Chim., 2013, 16, 222–228 CrossRef CAS.
- N. Inchaurrondo, P. Haure and J. Font, Desalination, 2013, 315, 76–82 CrossRef CAS.
- N. S. Inchaurrondo, P. Massa, R. Fenoglio, J. Font and P. Haure, Chem. Eng. J., 2012, 198, 426–434 CrossRef.
- P. R. Gogate and A. B. Pandit, Adv. Environ. Res., 2004, 8, 501–551 CrossRef CAS.
- J. Arana, E. T. Rendón, J. D. Rodrıguez, J. H. Melián, O. G. Dıaz and J. P. Pena, Chemosphere, 2001, 44, 1017–1023 CrossRef CAS PubMed.
- E. G. Garrido-Ramirez, M. V. Sivaiah, J. Barrault, S. Valange, B. K. Theng, M. S. Ureta-Zañartu and M. de la Luz Mora, Microporous Mesoporous Mater., 2012, 162, 189–198 CrossRef CAS.
- P. Bautista, A. F. Mohedano, N. Menendez, J. A. Casas and J. J. Rodriguez, Catal. Today, 2010, 151, 148–152 CrossRef CAS.
- K. Jíša, J. Nováková, M. Schwarze, A. Vondrová, S. Sklenák and Z. Sobalik, J. Catal., 2009, 262, 27–34 CrossRef.
- J. Pieterse, S. Booneveld and R. W. van den Brink, Appl. Catal., B, 2004, 51, 215–228 CrossRef CAS.
- S. Jiang, H. Zhang and Y. Yan, Catal. Commun., 2015, 71, 28–31 CrossRef CAS.
- G. Satishkumar, M. V. Landau, T. Buzaglo, L. Frimet, M. Ferentz, R. Vidruk, F. Wagner, Y. Gal and M. Herskowitz, Appl. Catal., B, 2013, 138, 276–284 CrossRef.
- L. Xiang, S. Royer, H. Zhang, J. Tatibouët, J. Barrault and S. Valange, J. Hazard. Mater., 2009, 172, 1175–1184 CrossRef CAS PubMed.
- C. Chiu, K. Hristovski, S. Huling and P. Westerhoff, Water Res., 2013, 47, 1596–1603 CrossRef CAS PubMed.
- F. Adam, J. Wong and E. Ng, Chem. Eng. J., 2013, 214, 63–67 CrossRef CAS.
- H. Kušić, N. Koprivanac and I. Selanec, Chemosphere, 2006, 65, 65–73 CrossRef PubMed.
- J. Faye, E. Guélou, J. Barrault, J. M. Tatibouët and S. Valange, Top. Catal., 2009, 52, 1211–1219 CrossRef CAS.
- H. Chen and W. M. Sachtler, Catal. Today, 1998, 42, 73–83 CrossRef CAS.
- K. Fajerwerg and H. Debellefontaine, Appl. Catal., B, 1996, 10, L229–L235 CrossRef CAS.
- K. Fajerwerg, J. N. Foussard, A. Perrard and H. Debellefontaine, Water Sci. Technol., 1997, 35, 103–110 CrossRef CAS.
- N. H. Phu, T. T. K. Hoa, N. van Tan, H. V. Thang and P. le Ha, Appl. Catal., B, 2001, 34, 267–275 CrossRef CAS.
- M. M. Treacy and J. B. Higgins, Collection of Simulated XRD Powder Patterns for Zeolites Fifth (5th) Revised Edition, Elsevier, 2007 Search PubMed.
- S. Chaliha and K. G. Bhattacharyya, Chem. Eng. J., 2008, 139, 575–588 CrossRef CAS.
- S. Chaliha and K. G. Bhattacharyya, J. Hazard. Mater., 2008, 150, 728–736 CrossRef CAS PubMed.
- P. L. Tan, Y. L. Leung, S. Y. Lai and C. T. Au, Appl. Catal., A, 2002, 228, 115–125 CrossRef CAS.
- D. Scarano, A. Zeccdhine, S. Bordiga, F. Geobaldo and G. Spoto, J. Chem. Soc., Faraday Trans., 1993, 89, 4123–4130 RSC.
- R. Szoztak, Molecular Sieves, Blackie Academic and Professional, London, 1998, p. 359 Search PubMed.
- S. Bordiga, R. Buzzoni, F. Geobaldo, C. Lamberti, E. Giamello, A. Zecchina, G. Leofanti, G. Petrini, G. Tozzola and G. Vlaic, J. Catal., 1996, 158, 486–501 CrossRef CAS.
- A. Ates, A. Reitzmann and G. Waters, Appl. Catal., B, 2012, 119–120, 329–339 CrossRef CAS.
- D. Meloni, R. Monaci, V. Solinas, G. Berlier, S. Bordiga, I. Rossetti, C. Oliva and L. Forni, J. Catal., 2003, 214, 169–178 CrossRef CAS.
- E. Dumitriu, V. Hulea, I. Fechete, A. Auroux, J. Lacaze and C. Guimon, Microporous Mesoporous Mater., 2001, 43, 341–359 CrossRef CAS.
- E. Guélou, J. Barrault, J. Fournier and J. Tatibouët, Appl. Catal., B, 2003, 44, 1–8 CrossRef.
- S. Zhang, X. Zhao, H. Niu, Y. Shi, Y. Cai and G. Jiang, J. Hazard. Mater., 2009, 167, 560–566 CrossRef CAS PubMed.
- H. R. Eisenhauer, J.–Water Pollut. Control Fed., 1964, 1116–1128 CAS.
- M. Neamtu, C. Zaharia, C. Catrinescu, A. Yediler, M. Macoveanu and A. Kettrup, Appl. Catal., B, 2004, 48, 287–294 CrossRef CAS.
- J. A. Zazo, J. A. Casas, A. F. Mohedano and J. J. Rodriguez, Appl. Catal., B, 2006, 65, 261–268 CrossRef CAS.
- F. Adam, J. Andas and I. A. Rahman, Chem. Eng. J., 2010, 165, 658–667 CrossRef CAS.
- A. Santos, P. Yustos, A. Quintanilla, F. Garcia-Ochoa, J. A. Casas and J. J. Rodriguez, Environ. Sci. Technol., 2004, 38, 133–138 CrossRef CAS PubMed.
- G. Centi, S. Perathoner, T. Torre and M. G. Verduna, Catal. Today, 2000, 55, 61–69 CrossRef CAS.
- M. Dükkancı, G. Gündüz, S. Yılmaz and R. V. Prihod Ko, J. Hazard. Mater., 2010, 181, 343–350 CrossRef PubMed.
- T. D. Nguyen, N. H. Phan, M. H. Do and K. T. Ngo, J. Hazard. Mater., 2011, 185, 653–661 CrossRef CAS PubMed.
- M. Pera-Titus, V. García-Molina, M. A. Baños, J. Giménez and S. Esplugas, Appl. Catal., B, 2004, 47, 219–256 CrossRef CAS.
- H. Xu, D. Zhang and W. Xu, J. Hazard. Mater., 2008, 158, 445–453 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |