SiO2–Ti0.98In0.01Nb0.01O2 composite ceramics with low dielectric loss, high dielectric permittivity and an enhanced breakdown electric field†
Abstract
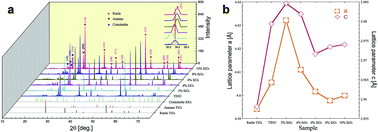
SiO2–Ti0.98In0.01Nb0.01O2 (SiO2–TINO) composite ceramics were synthesized by solid-state sintering methods. The dielectric loss can be lowered and the breakdown electric field can be greatly enhanced by doping 2 mol% SiO2 into TINO ceramics, while preserving the relatively high dielectric permittivity (∼30 000). The microstructure and electrical response of SiO2–TINO composite ceramics were studied to investigate the reasons for these good dielectric properties. The lower dielectric loss and enhanced breakdown electric field were attributed to the embedding of SiO2 in the TINO grain boundaries, which induced a drop in the potential and a blocking effect around the grain boundaries for the 2 mol% SiO2–TINO composite ceramic. The results provide a way to lower the dielectric loss and enhance the breakdown voltage by tuning the grain boundary.

 Please wait while we load your content...
Please wait while we load your content...