Mechanical stability and rheology of lithium–calcium-based grease containing ZDDP
Tiejun Shenabc,
Daxi Wang*ab,
Jimmy Yund,
Qinglian Liuc,
Xinghua Liuab and
Zhongxiao Penge
aState Key Laboratory of Heavy Oil Processing in China University of Petroleum, Beijing 102249, P. R. China. E-mail: sjingshi@126.com; wdxi@cup.edu.cn; Tel: +86 13821480747
bCollege of Science in China University of Petroleum, Beijing 102249, P. R. China
cLubricant Tianjin Company, SINOPEC CORP, 5 Huagong street Hangu, Tianjin 300480, P. R. China
dSchool of Chemical Engineering, University of New South Wales, Sydney 2052, Australia
eSchool of Mechanical & Manufacturing Engineering, The University of New South Wales, Sydney 2052, Australia
First published on 12th January 2016
Abstract
Although lubricating greases with zinc dialkyl-dithiophates (ZDDP) have been used widely, the influence of ZDDP on the rheological properties of lithium–calcium-based greases (LCBG) has not been reported. This work investigated the effects of ZDDP on the rheology and mechanical stability of lithium–calcium-based greases (LCBG) by means of scanning electron microscopic (SEM) analysis, rheology characterization and roll stability test. It was found that the thickener's base grease fiber containing ZDDP becomes looser, and the effects of ZDDP on low-alkali LCBG are greater than that on low-acid LCBG. Thereby, the values of the viscoelastic functions in the linear viscoelastic region, the shear stress and mechanical stability of LCBG decrease with the increase in ZDDP concentration. Due to the fibrous instability of the thickener above 60 °C, considerable shortening of the thickener's fibers and increasing penetration values after shearing of the grease in a roll stability tester at 75 °C, as well as transformation from shear thinning to shear thickening at the shear rate of 1800 s−1 and 80 °C, were observed. The maximum recommended ZDDP concentrations related to mechanical stability and resistance under working conditions are below 4 wt% for low-alkali grease and 5 wt% for low-acid grease. Additionally, the saturated adsorption of 3 wt% ZDDP on the soap fibers can lead to sufficient ZDDP migration to the polar metallic surface, which indicates the optimized tribological effect of ZDDP on LCBG. This study provides a promising formulation range of around 3% to 5 wt% ZDDP in low-acid grease.
1. Introduction
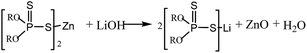
Lubricating grease is widely used in many applications to reduce the wear and friction in bearings and other rolling elements. Owing to its semisolid character, greases stay in the mechanically moving parts and act as a seal without a sump to prevent contaminants in solids or liquids from entering the system. Most functional properties depend on the ability of the grease to flow under external force, as well as its shear and viscosity stability under different temperatures and pressures. In particular, structural viscosity stability in operation conditions is critical to the long-term service life of grease-lubricated roller bearings. Due to mechanical forces between the rolling parts of the bearing, poor mechanical stability leads to degradation of grease consistency and results in the leakage of grease through seals or, at worst, a total failure of the bearing.1 Today, greases thickened with soaps of metals, mainly lithium (about 65% of world production), form the largest group of greases. The second largest group of greases includes those thickened with calcium soaps, which have excellent mechanical stability and anti-water performance compared to other main groups of grease. As pointed out in,2 in most cases, the thickener soaps (metal salt of 12-hydroxystearate) of metals crystallize in the form of long twisted ribbons, and the mineral oil is wrapped in a three-dimensional gelling network, in which a number of interaction forces, such as electrostatic repulsions, van der Waals forces, and steric repulsions,3 are involved. The rheological behaviors of lubricating grease, including the yield stress, shear thickening,4 shear thinning,5 and linear or nonlinear viscoelasticity, depend considerably on many factors, among which the microstructure and interaction forces of the gelling network are two main contributors. Some researchers discovered that the mechanical durability and the stability of the microstructure of the thickener in lubricating grease determine the friction reduction, the protection of the lubricated surfaces, as well as the grease performance during mechanical loading in the friction node at ultimately high working temperatures.6,7 Therefore, study on the relationship between the microstructure and the rheological behaviors is essential to elucidate the effect on the mechanical properties of lubricating grease in bearing lubrication.In most cases, it is desirable to incorporate different additives into a grease formulation that can modify some functional properties of the base oil in the grease at high temperature and pressure. ZDDP has been widely used as the best anti-wear and anti-oxidant additive for engine lubricants for years, and no single replacement acts more effectively.8,9 Meanwhile, to meet different technical and environmental requirements for different parts between movable joints without a sump, lubricating grease with ZDDP has been used widely. In addition, it has been found that the anti-water performance of LCBG is improved with low ZDDP content in low-acid grease.10 However, due to the interaction with lithium 12-hydroxystearate, ZDDP is adsorbed on the soap fiber,11 which has a profound effect on the oil separation,12 the dropping point and penetration properties of the grease.13 Therefore, the rheological behaviors and mechanical stability of LCBG are largely influenced by ZDDP.
Although a large number of investigations were carried out to study the rheological behaviors of the lubricating grease, most of them focused particularly on the characterization of products through rheometry.14–16 Generally speaking, the rheological behavior of lubricating grease mainly depends upon the soap concentration,17 viscosity18 and type of base oil,17,19 manufacturing process, and thickening agent of the grease.20–23 Due to the influence of the fiber structure on the LCBG rheological behaviors as reported in,24 it is speculated that ZDDP may alter the rheological properties of LCBG by affecting the soap and gelling network. In turn, the rheological behavior of the grease provides information on interactions between the gelling network and the media. Furthermore, insight into the influence of ZDDP on the rheological data of the grease over a wide range of shear stress or shear rate is very useful to predict the mechanical stability. However, when reviewing available literature on the rheological behaviors of greases, the authors found that there is a lack of comprehensive studies on the combined effects of ZDDP and stearic acid on the rheological behaviors, mechanical stability and microstructure of grease.
Therefore, in this study, to minimize the potential negative effects of ZDDP on grease when adding a slice of stearic acid into LCBG, the effects of different ZDDP contents on the mechanical stability and rheological properties of LCBG were experimentally investigated; the relationships between them were interpreted from a microstructure standpoint. The ultimate goal of this work is to develop promising anti-wear lubricating grease with excellent mechanical stability.
2. Experimental section
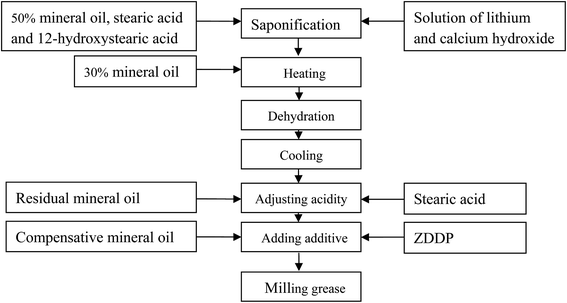
2.1. Materials and preparation of grease
Two mineral oils, i.e. 150 BS with kinematic viscosity of 485 cSt at 40 °C and 500 SN with kinematic viscosity of 100 cSt at 40 °C (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, weight ratio), were selected as the base oils of the lubricating greases because they are widely used in lubricating grease. As shown in Fig. 1, 12-hydroxystearic acid and stearic acid, in the weight ratio of 3
3, weight ratio), were selected as the base oils of the lubricating greases because they are widely used in lubricating grease. As shown in Fig. 1, 12-hydroxystearic acid and stearic acid, in the weight ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,24 were added to the mineral oil to make 40–50 wt% at 80 °C. The mixture was heated until all fatty acids were melted. A solution with 3
1,24 were added to the mineral oil to make 40–50 wt% at 80 °C. The mixture was heated until all fatty acids were melted. A solution with 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of lithium hydroxide to calcium hydroxide24 was then introduced and heated for 2 hours under mild agitation to evaporate the water. Another portion (15–30 wt%) of mineral oil was added at 180 °C, and the solution was further heated to 210 °C for 5–10 minutes to dehydrate the soap. When the grease was cooled to 120 °C, a slice of stearic acid was introduced to yield the low-acid grease with the fatty acid content of 0.028 mg oleic acid per gram (named as grease A), or else the low-alkali grease with 0.02 wt% NaOH (indicating 0.012 wt% surplus LiOH in grease) was obtained (grease B). Finally, the residual mineral oil was added. As the greases were cooled to 80 °C, the commercial ZDDP with 0–7 weight percent and compensative mineral oil of 500 SN (to obtain the same concentration of thickener) were added into these two resulting lubricating greases. The soluble ZDDP contains 15 wt% isooctyl and 85 wt% isobutyl in this study. Whether with or without ZDDP, all samples with 11.0 wt% thickener were milled thrice using 3-roll mill equipment at room temperature until they were thoroughly homogenous and had a smooth, paste-like texture. Furthermore, to minimize the initial deviations of tests described in Section 2.2 and to standardize the initial state of the tested greases, as previously described, all samples were prepared using the same procedures.
1 molar ratio of lithium hydroxide to calcium hydroxide24 was then introduced and heated for 2 hours under mild agitation to evaporate the water. Another portion (15–30 wt%) of mineral oil was added at 180 °C, and the solution was further heated to 210 °C for 5–10 minutes to dehydrate the soap. When the grease was cooled to 120 °C, a slice of stearic acid was introduced to yield the low-acid grease with the fatty acid content of 0.028 mg oleic acid per gram (named as grease A), or else the low-alkali grease with 0.02 wt% NaOH (indicating 0.012 wt% surplus LiOH in grease) was obtained (grease B). Finally, the residual mineral oil was added. As the greases were cooled to 80 °C, the commercial ZDDP with 0–7 weight percent and compensative mineral oil of 500 SN (to obtain the same concentration of thickener) were added into these two resulting lubricating greases. The soluble ZDDP contains 15 wt% isooctyl and 85 wt% isobutyl in this study. Whether with or without ZDDP, all samples with 11.0 wt% thickener were milled thrice using 3-roll mill equipment at room temperature until they were thoroughly homogenous and had a smooth, paste-like texture. Furthermore, to minimize the initial deviations of tests described in Section 2.2 and to standardize the initial state of the tested greases, as previously described, all samples were prepared using the same procedures.
2.2. Experimental procedures and analytical methods
Morphological observations of the greases were carried out with the assistance of a scanning electron microscope (SEM), model S-3400N (Hitachi, Japan). Each sample was placed onto a copper grid and immersed in hexane for 90 min to extract the oil. The immersion process was repeated several times to ensure the complete extraction of the oil.25 Then, the sample was dried at room temperature and coated with gold. Micrographs of the sample were acquired at an acceleration voltage of 20 kV and a magnification of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×.
000×.
The rheological characterization of the lubricating grease was conducted on an automatic rotational-controlled rheometer (Anton-Paar MCR 302, Germany) with a plate–plate geometry having a gap size of 1 mm and a diameter of 25 mm. All samples had the same thermal treatments before the rheological tests. At least three replicates of each test were performed on fresh samples according to the following experimental procedures.
In dynamic oscillatory tests, the strain sweep in the range 1.0 × 10−4 to 1.0 was performed at 10 rad s−1 and 25 °C. The values of storage modulus G′ and the loss modulus G′′ as a function of strain were recorded. In addition, the viscoelastic properties of those greases were studied in the oscillatory shear mode over a frequency range of 0.4 to 400 rad s−1 in the linear viscoelastic region.
To study the flow properties of the prepared greases, continuous shear experiments were carried out. All the flow curves were obtained by applying a stepwise ramp of increasing shear rates/stresses and waiting for sufficient times at each step, almost enough to attain a steady-state condition within the range of studied shear rate/stress. Firstly, viscous flow measurements were performed within a temperature ranging from 25 to 80 °C and at a shear rate ranging from 0.1 to 1500 or 1800 s−1, followed by a back shear rate ranging from 1500 or 1800 to 0.1 s−1. Meanwhile, the influence of the shear rate and temperature on the thixotropy of the greases was investigated under the steady shear rate of 500 s−1 at 25 °C, shear rate of 500 s−1 at 80 °C, shear rate of 1800 s−1 at 25 °C and shear rate of 1800 s−1 at 80 °C, respectively, for 10 min. Additionally, to study the rheological properties of the greases under a low shear rate, measurements of shear stress flow were performed at 25 °C, with a shear rate ranging from 0.01 s−1 to 10 s−1 in a logarithmic scale, and followed by a shear rate from 10 s−1 to 20 s−1 in a linear scale.
To characterize the mechanical stability of the greases, the samples were tested in a roll stability tester (model 1210 MFG, RIGOSHA, Japan), according to the ASTM D1831 standard. In the roll stability test, a small sample (50 g) of grease was milled in a cylindrical chamber by a heavy roller for 24 h at 75 °C. Both the pre-tested and post-tested penetration indexes were determined based on the ASTM D1403 standard and using the Sinopec penetrometer with a one-quarter cone geometry. The one-quarter-scale penetration values were converted into the equivalent full-scale cone penetration values, which are in accord with ASTM D217. In this test, a change in grease consistency after the roll stability test was reported as the absolute change in penetration, mm. In order to characterize the influence of the roll stability test on the fiber structure of the greases, morphological observations of the greases were also conducted using a SEM after 7 days of the roll stability test.
3. Results and discussion
3.1. Gelling network
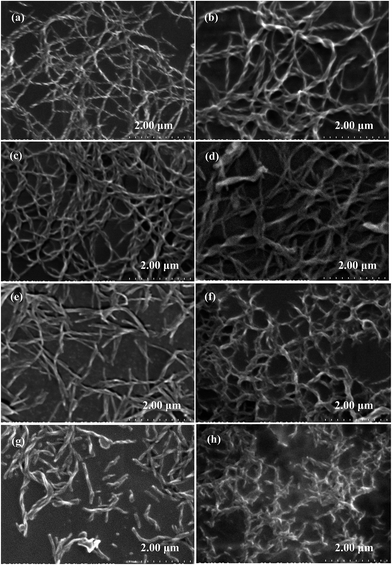
Lubricating grease is a colloid with a soap thickener in mineral or synthetic oil that shows conspicuously non-Newtonian characteristics. The lithium and calcium hydroxy stearate molecules, acting as the metal soap thickener, are composed of carboxylate, a hydroxyl group, and a non-polar aliphatic group. It is widely recognized that the soap crystallites are formed by the ionic strength, hydrogen bond, van der Waals forces, and so on, after being dispersed in the mineral oils.Fig. 2 shows the effects of additive on the fiber structures of the soap before and after the roll stability test. It can be clearly seen that the metal soap appears as long twisted fibers in the greases. These soap fibers are prone to disperse randomly within a given volume and form a three-dimensional gelling network with the mineral oil in it. According to the two manners of trapping the base oil molecules, i.e. the direct sorption of the oil by non-polar ends of the soap molecule, and the penetration of the base oil into the interlacing structure of the soap fiber,26 the mineral oil thus can exist in three possible forms, which include free oil among the gelling networks, capillary oil among the soap fibers, and swelled oil among the alkyls of the soap fiber molecules.27 Fig. 3 shows that, under a similar magnification, the mean diameters of the soap fibers were 108 ± 13 nm and 145 ± 28 nm for grease A and grease B, respectively. Grease A has a relatively more compact fiber structure with a larger ratio of length to diameter than grease B. This indicates that, when compared with grease B, the network of grease A was more stable and attracted the base oil molecules more firmly.
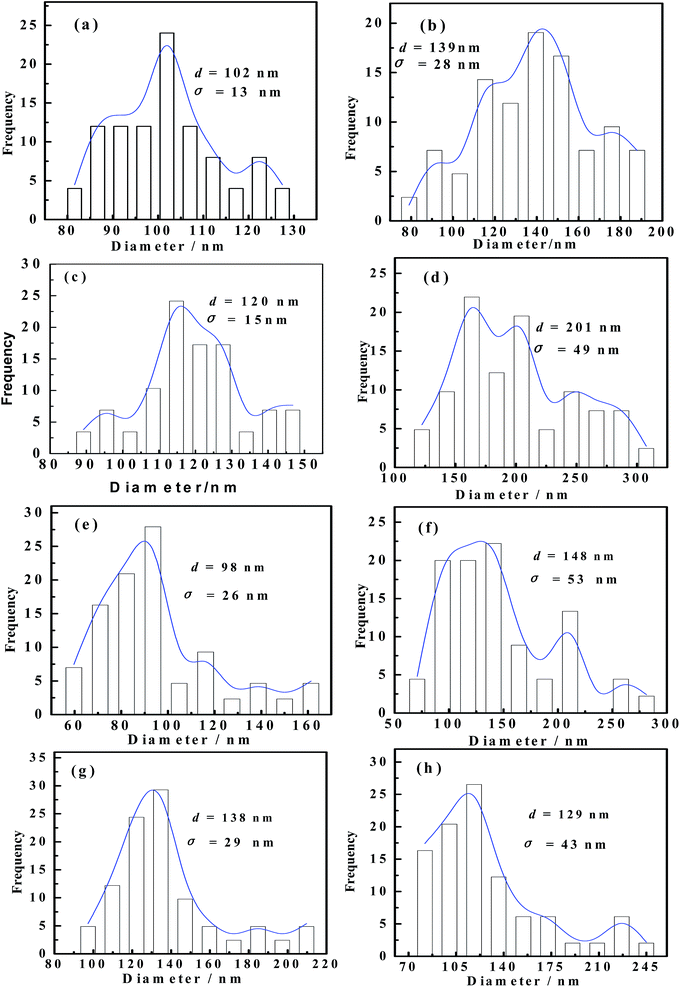 | ||
| Fig. 3 The diameter distributions of soap fiber images in Fig. 2: (a) for Fig. 2a, (b) for Fig. 2b, (c) for Fig. 2c, (d) for Fig. 2d, (e) for Fig. 2e, (f) for Fig. 2f, (g) for Fig. 2g and (h) for Fig. 2h (where d is the mean diameter value of fiber, and σ is the standard deviation of diameters). | ||
The additives introduced during the cooling stage of grease formation and homogeneously dispersed in the matrix are often used to enhance some functional properties of the base oil in the grease, such as oxidation, load bearing, anti-wear, anti-corrosion, and anti-rust.28 The polar head, which contains zinc, phosphorus, oxygen and sulfur in ZDDP molecules, is first bound to the soap fibers, and the non-polar hydrocarbon chains attached to ZDDP hold the oil;11 thus ZDDP influences the soap thickener and the network structure either by changing the solubility of the soap in the base oil or by affecting the fiber-bridging process. The average diameters of the soap fibers were estimated to be 108 ± 13 nm, 145 ± 28 nm, 120 ± 15 nm and 201 ± 49 nm from the SEM images of greases A without ZDDP, B without ZDDP, A with 5 wt% ZDDP and B with 5 wt% ZDDP, respectively (Fig. 3a–d). It can be seen that the ZDDP-doped grease had a looser fiber structure with larger and sparser fibers than the one without ZDDP, and the influence of ZDDP on the fiber structure of grease B is greater than on that of grease A. Thus, it is proposed that ZDDP causes subsequent changes to the network stability and physical behavior of the grease, especially the rheological behavior and mechanical stability.
3.2. Rheological characterization
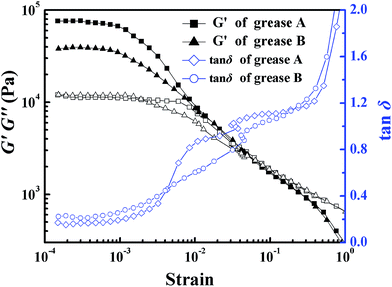
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ curves and shear stress values (not shown in Fig. 4) were measured by using a dynamic rotating rheometer. The results showed that at low strains, the dominant modulus was the storage modulus, G′, which indicated that the values of the elastic properties were greater than those of the viscous properties, and a strong gel network had been formed. The relationship between the storage modulus and the loss modulus was linear until the critical strain of 0.0028 was obtained. At this critical strain, the shear stress of grease A and grease B was 118.38 Pa and 46.16 Pa, respectively. The region before the critical strain occurs is known as the linear viscoelastic region. In this region, the response (e.g., stress) at any time is directly proportional to the value of the initiating signal (e.g., strain).18 Beyond the critical strain, G′ started to decrease while G′′ remained unchanged, and the loss tangent, tan
δ curves and shear stress values (not shown in Fig. 4) were measured by using a dynamic rotating rheometer. The results showed that at low strains, the dominant modulus was the storage modulus, G′, which indicated that the values of the elastic properties were greater than those of the viscous properties, and a strong gel network had been formed. The relationship between the storage modulus and the loss modulus was linear until the critical strain of 0.0028 was obtained. At this critical strain, the shear stress of grease A and grease B was 118.38 Pa and 46.16 Pa, respectively. The region before the critical strain occurs is known as the linear viscoelastic region. In this region, the response (e.g., stress) at any time is directly proportional to the value of the initiating signal (e.g., strain).18 Beyond the critical strain, G′ started to decrease while G′′ remained unchanged, and the loss tangent, tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ, started to increase, which suggested the breakdown of structure within the gel network of the grease. Thereby, the results of the frequency sweeps about the undamaged microstructural network of these greases should be obtained from the linear viscoelastic region. Then, a certain crossover point with the shear stress of 208.5 Pa for grease B and 157.8 Pa for grease A was obtained, where G′ equaled G′′ and hence tan
δ, started to increase, which suggested the breakdown of structure within the gel network of the grease. Thereby, the results of the frequency sweeps about the undamaged microstructural network of these greases should be obtained from the linear viscoelastic region. Then, a certain crossover point with the shear stress of 208.5 Pa for grease B and 157.8 Pa for grease A was obtained, where G′ equaled G′′ and hence tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ = 1, subsequently with greater G′′ than G′ from there on, which indicates that the lubricating grease flowed like fluids. Similar to the yield stresses of grease A and grease B in Table 1, it was found that the yield stress and the G′ of grease A were larger than those of grease B at the critical strain, which reveals that the gel network of grease A was stronger than that of grease B. However, the shear stress of grease B was larger than that of grease A at the crossover points, and the values of G′′ of grease B were slightly larger than those of grease A in the linear viscoelastic region. As a result, the inner friction of grease A was lower, and the energy consumption was less than that of grease B.
δ = 1, subsequently with greater G′′ than G′ from there on, which indicates that the lubricating grease flowed like fluids. Similar to the yield stresses of grease A and grease B in Table 1, it was found that the yield stress and the G′ of grease A were larger than those of grease B at the critical strain, which reveals that the gel network of grease A was stronger than that of grease B. However, the shear stress of grease B was larger than that of grease A at the crossover points, and the values of G′′ of grease B were slightly larger than those of grease A in the linear viscoelastic region. As a result, the inner friction of grease A was lower, and the energy consumption was less than that of grease B.
| τ0 ± σ (Pa) | K ± σ (Pa sn) | N ± σ | SHL ± σ (Pa s−1) | |
|---|---|---|---|---|
| Grease A | 223.2 ± 1.1 | 209 ± 1.6 | 0.328 ± 0.011 | 3.29 × 105 ± 0.05 × 105 |
| Grease B | 211.9 ± 1.3 | 301.3 ± 1.4 | 0.277 ± 0.024 | 4.92 × 105 ± 0.08 × 105 |
The results of strain scanning for grease A and grease B with different ZDDP concentrations of 1, 2, 3, 4, 5, 6, and 7 wt% were similar to those in Fig. 4, and all the values of the critical strain were within 0.003. Therefore, as shown in Fig. 5a–d, the angular frequency sweeps were obtained in the linear region with the ZDDP concentrations of 0, 3 and 7 wt%. Generally, the value of G′ was found to be greater than G′′ in all the angular frequencies, which is indicative of the formation of a strong gel network with or without ZDDP. This supports the idea that the lubricating greases are highly structured systems, as has been detected by SEM. However, in Fig. 5c and d, the values of G′ and G′′ decreased with the increase of ZDDP concentration, which is more prominent for samples with higher ZDDP concentration, especially for grease B. Meanwhile, compared to quite similar values on the loss tangent of grease A in the entire frequency range studied (Fig. 5a), grease B showed significant decrease in the values of the loss tangent with the increase of ZDDP concentration, along with the higher relative elastic characteristics in Fig. 5b. As pointed out by others,19 the increasing attraction forces between the lubricating oil and thickener result in the decreasing values of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ. Therefore, the addition of ZDDP increased the attraction forces between the oil and the thickener (the solvency of thickener in the lubricating oil), which led to a lower volume fraction of fibers forming the network and resulted in a looser fiber structure with larger and sparser fiber (Fig. 2c and d). Thereby, the grease showed lower values of G′ and G′′ with increased ZDDP concentration, which is indicative of weaker microstructural network. However, because of the common ion effect, the surplus stearic acid in the oil dampened the increase of the solvency between the metal soap and the base oil in grease A. Therefore, both G′ and G′′ of grease B decreased faster than those of grease A with the increase of ZDDP concentration. Especially, Fig. 5c shows that the G′′ values of the sample with 3% ZDDP are higher than those of grease A with 0% and 7% ZDDP at low angular frequency, indicating that the microstructural network of grease A with 3% ZDDP is quite different from that of other samples, which is discussed in Section 3.2.3.
δ. Therefore, the addition of ZDDP increased the attraction forces between the oil and the thickener (the solvency of thickener in the lubricating oil), which led to a lower volume fraction of fibers forming the network and resulted in a looser fiber structure with larger and sparser fiber (Fig. 2c and d). Thereby, the grease showed lower values of G′ and G′′ with increased ZDDP concentration, which is indicative of weaker microstructural network. However, because of the common ion effect, the surplus stearic acid in the oil dampened the increase of the solvency between the metal soap and the base oil in grease A. Therefore, both G′ and G′′ of grease B decreased faster than those of grease A with the increase of ZDDP concentration. Especially, Fig. 5c shows that the G′′ values of the sample with 3% ZDDP are higher than those of grease A with 0% and 7% ZDDP at low angular frequency, indicating that the microstructural network of grease A with 3% ZDDP is quite different from that of other samples, which is discussed in Section 3.2.3.
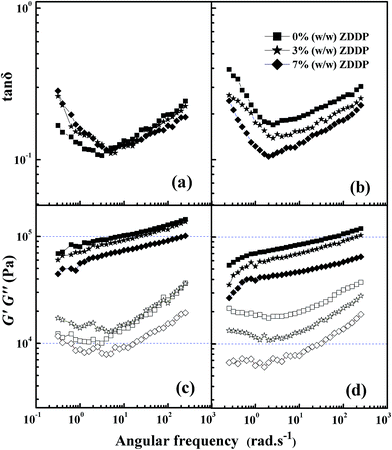 | ||
| Fig. 5 Angular frequency sweeps at various ZDDP concentrations, where (a) and (c) grease A, (b) and (d) grease B (filled symbols, G′; empty symbols, G′′). | ||
The ‘‘plateau’’ modulus, G0N, is a characteristic parameter for the intermediate plateau region and is defined as the extrapolation of the contribution of the level of entanglements to G′ at high frequencies.29 It could be considered as a measure of the aggregation number among the dispersed structural units or the density of physical entanglements, and consequently, is related to the strength of the microstructural network. A straightforward method to estimate G0N from the loss tangent was selected in this work.29
G0N = [G′]tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ → minimum δ → minimum
| (1) |
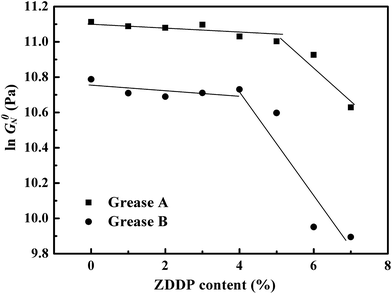
For all the lubricating greases studied, the experimental values of the plateau modulus are shown in Fig. 6. As could be observed, the values of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G0N generally decreased with the increase of weight fraction, which was confirmed to form a weaker gel network as the ZDDP content increased.17 Meanwhile, there was a dramatic change of the slope for the plot of ln
G0N generally decreased with the increase of weight fraction, which was confirmed to form a weaker gel network as the ZDDP content increased.17 Meanwhile, there was a dramatic change of the slope for the plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G0N versus ZDDP content, which was at the critical concentration of around 5 wt% for grease A and 4 wt% for grease B. A much higher ZDDP susceptibility of the lubricating grease, with the ZDDP concentration above 5 wt% for grease A and 4 wt% for grease B, was thus deduced, and the influence of ZDDP on the strength of the microstructural network of grease B was confirmed to be larger than on that of grease A. Taking the weak structural network at higher ZDDP concentration mentioned above into account, greases with ZDDP exhibited a lower level of cross-linked points and showed less extent of elastic characteristics than those without ZDDP. Moreover, the hydrogen bonds between fibers in grease A was improved by surplus stearic acid. Accordingly, grease A had a higher value of ln
G0N versus ZDDP content, which was at the critical concentration of around 5 wt% for grease A and 4 wt% for grease B. A much higher ZDDP susceptibility of the lubricating grease, with the ZDDP concentration above 5 wt% for grease A and 4 wt% for grease B, was thus deduced, and the influence of ZDDP on the strength of the microstructural network of grease B was confirmed to be larger than on that of grease A. Taking the weak structural network at higher ZDDP concentration mentioned above into account, greases with ZDDP exhibited a lower level of cross-linked points and showed less extent of elastic characteristics than those without ZDDP. Moreover, the hydrogen bonds between fibers in grease A was improved by surplus stearic acid. Accordingly, grease A had a higher value of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G0N than grease B.
G0N than grease B.
τ = τ0 + k![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) n. n.
| (2) |
![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/char_e0a2.gif) are the consistency, shear thinning indexes and the shear rate, respectively.18 Table 1 shows that the lower yield stress and the larger SHL of grease B contributed to the higher consistency factor. Some researchers15,16,20 pointed out that the yield stress value plays an important role in the network. The grease exhibits a solid-like behavior below this value, while the gelling network structure was deformed as long as the yield exceeded this threshold, which mainly resulted from the well-known elastic deformation of greases. Therefore, the elasticity of grease A was larger than that of grease B. Additionally, the flow index (n) of grease B was lower than that of grease A, which is representative of the typical yielding behavior shown by grease B.19
are the consistency, shear thinning indexes and the shear rate, respectively.18 Table 1 shows that the lower yield stress and the larger SHL of grease B contributed to the higher consistency factor. Some researchers15,16,20 pointed out that the yield stress value plays an important role in the network. The grease exhibits a solid-like behavior below this value, while the gelling network structure was deformed as long as the yield exceeded this threshold, which mainly resulted from the well-known elastic deformation of greases. Therefore, the elasticity of grease A was larger than that of grease B. Additionally, the flow index (n) of grease B was lower than that of grease A, which is representative of the typical yielding behavior shown by grease B.19
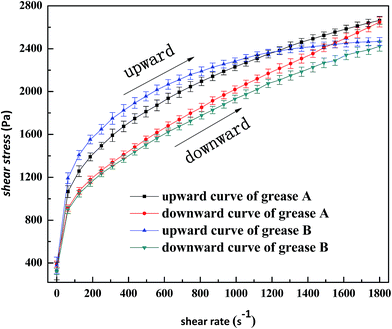
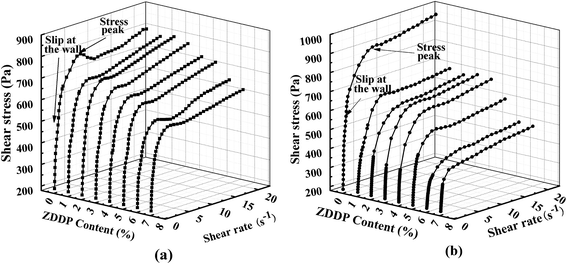
In Fig. 8, under low shear rate, there was a peak on each curve around the shear rate 5 s−1. Furthermore, the peak intensity of grease A without ZDDP was greater than that of grease B without ZDDP. The value of critical stress defines the plateau stress value or the ‘‘classic’’ yield stress value.32 These peaks are attributed to elastic overshoot32 and related to some elastic behavior of the sample. They also represent the force that may be required to destroy the structure, release the capillary oil from the gelling networks, and make the grease flow.3 Thereby, the shear flow process takes the main role in the structural breakdown of the gelling network. The larger the interaction forces of the gelling network structure of the grease are, the greater the peak intensity will be.33 From a microstructural point of view, by adding the surplus stearic acid, grease A yielded a relatively more compact fiber structure (Fig. 2 and 3), which would result in a more stable network. However, the surplus stearic acid also lowered the solvency of the metal soap in the base oil, which induced the decrease of the proportion of both the capillary and swelled oil and the increase of dissociative oil, and led to lower viscosity of grease A. As a result, the shear stress of grease A without ZDDP was lower than that of grease B without ZDDP at the stress peak, as shown in Fig. 8. Additionally, there were some influences of wall slip that contributed to a reduction in the yield stress and led to a difference between the shear stresses of grease A and grease B at the stress peak; this is discussed later in Section 3.2.3.
 | (3) |
Moreover, the surplus stearic acid led to an ideal fiber crystallization structure for grease A. As a result, the stress peak and shear stress of grease B decreased at a larger scale than that of grease A when 1 wt% ZDDP was added (Fig. 8b). It is also observed that the stress peaks of both greases A and B slightly decreased with the ZDDP concentration increasing from 1 wt% to 3 wt%, followed by a gradual decrease with more ZDDP addition, indicating that the saturated adsorption of ZDDP on the soap fibers occurred when the ZDDP concentration was 3 wt%. In case of grease applications, there are two types of polar surfaces, that is, the metal surface and thickener fiber surface.11 Then, the adsorption of ZDDP on the metal surface competes with the thickener fiber, and the saturated adsorption of ZDDP on the soap fibers made ZDDP sufficiently migrate to the polar metallic surface. Thus, in our previous study,34 it was found that, to improve the antiwear ability, the optimum concentration of ZDDP is approximately 3 wt% in the base grease.
Paszkowski27 and Balan and Franco35 noticed that rough surfaces contributed to a reduction in wall effects, which was consistent with existing theory.3 Accordingly, when a smooth surface is used, the displacement of the dispersed phase away from the boundary may give rise to some slip at the wall and may result in a wrong value of yield stress.27 As shown in Fig. 8a and b, with the flow curve shifting towards lower stresses, both greases A and B showed wall slip at low shear rate below 1 s−1, and the wall slip was gradually weakened with the increase of ZDDP concentration. Moreover, the wall slip of grease A was more notable than that of grease B. The differences in stress can be explained by the fact that the materials were able to absorb grease thickener particles on the rheometer surface, which resulted in the formation of a certain grease volume with reduced thickener concentration. This volume was characterized by a substantially lower structural viscosity than the rest of the grease.27 In the case of grease A, the absorbability of thickener particles was enhanced by the presence of surplus stearic acid. As a result, a clear local increase in the thickener particle concentration of grease A occurred in the vicinity of the wall. The structural viscosity of grease A in the boundary layer was lower than that of grease B. Nevertheless, due to a competition of absorbability among thickener particles and ZDDP on the rotational rheometer surface, ZDDP additive with higher absorbability decreased the adsorption quantity of thickener particles but enhanced the anti-wear of the grease,34 which led to a gradual reduction in the wall slip with the increase of ZDDP. In turn, the absorption of ZDDP on the metal surface was compensated by the enhanced absorbability of the thickener, and hence the anti-wear ability of ZDDP was improved in grease A because of the presence of the surplus stearic acid.34
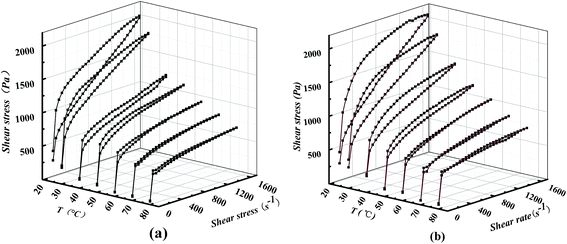
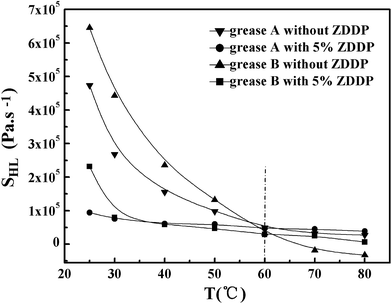
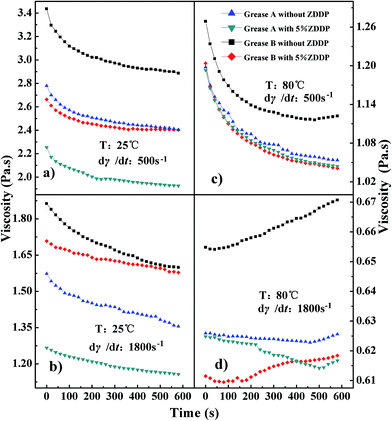
It is widely believed that the gelling network is formed through Brownian motion by the interactions of the ion force, van der Waals and the hydrogen bond among the fibers and dispersant during rest.2,3,36 Now that the destroying rates are greater than the restoring rates of the fibrous contacts,37 the shear breaks down part of this structure. The higher the temperature, the more intensive the Brownian motion, and the faster the kinetic rates of destroying and restoring the fibrous contacts. As shown in Fig. 9 and 10, the shear stress and the thixotropy of the samples sharply decreased when the temperature increased from 25 to 60 °C. Van der Waals attractions were mainly responsible for sorption of the oil by non-polar ends of the soap molecule, fiber entanglement and fiber grouping,3 which decreased quickly with the increase of temperature up to 60 °C. When the temperature surpassed 60 °C, the shear stress and the thixotropy of the samples slowly decreased. Some researchers reported that a non-monotonic evolution of the shear stress with increasing shear rate was observed at temperatures above 60 °C, which falls in a dynamically non-stable region and favors the fracture of the sample at sufficiently high shear rates.33 Part of the stable fibers mainly formed by the ion force (a strong interaction) would be broken. As a result, the obtained SHL values of samples were almost zero or negative. Probably, the hysteresis technique is not suitable for the analysis of the thixotropy of the samples at temperatures above 60 °C. Additionally, Fig. 10 also shows that the addition of either ZDDP or stearic acid led to a decrease of the SHL below 60 °C and an increase of the SHL beyond 60 °C. Therefore, when the shearing temperature is below 60 °C, ZDDP and the stearic acid mainly decrease van der Waals forces among gelling networks; when the temperature is above 60 °C, they mainly alter the fibrous ion crystal structure of the soap.
As shown in Fig. 9b, the double curves of grease B at 60 °C, 70 °C and 80 °C was interestingly in the shape of the number “8”. The ascending curve turned from above the descending curve to below the descending curve at the intersection point, which is indicative of transformation for the variation of the fluid structure. Meanwhile, the critical shear rates of the intersection points decreased with temperature increment. For example, the critical shear rates were approximately 1000, 300 and 200 s−1 at 60, 70 and 80 °C, respectively. These results indicate that the cross-linked thickener microstructure underwent disintegration, forming smaller aggregates and single flow units.2 Especially, the sol was formed by the majority of isolated fibers at critical shear rate, followed by the transformation of the structure of fluid with the fracture of fibers. The high temperatures resulted in the easy transformation of the fluid structure. These results can also be confirmed by the phenomena shown in Fig. 11. For the same grease at the same shear time, the viscosity curves in Fig. 11b and c were lower than those in Fig. 11a, indicating that the higher the shear rate and temperature, the easier it is to destroy the gelling networks. On the other hand, only grease B showed the critical shear time of about 500 s at the shear rate of 500 s−1 and temperature 80 °C. Before reaching the critical shear time, the viscosity was continuously reduced; the viscosity increased thereafter (Fig. 11b). This critical shear time indicated the transition from shear thinning to shear thickening. At the shear rate of 1800 s−1 at 80 °C, the critical shear times for grease B without and with 5% ZDDP were 60 and 180 s, while for grease A, they were 460 and 500 s, respectively (Fig. 11d). These results indicate that ZDDP and stearic acid delayed the transformation to shear thickening from shear thinning at a higher shear rate. Reasons for these results are explained as follows. On one hand, as long as the shear time was above the critical time, the fibers became loose or even ruptured, the effective phase volume increased, and more base oil was adsorbed onto the soap fibers, along with the decrease of proportion of the plastic base oil.4 These results contributed to the turnover of the shear thickening.4 On the other hand, not only could the stearic acid keep the soap fibers from being sheared loosely and ruptured by improving the structure stability, but the ZDDP and stearic acid with polar head groups were also easily absorbed onto the new surface amongst the ruptured soap fibers and inhibited the base oil from being adsorbed onto the soap fibers. Thereby, the turning of shear thickening was depressed, while this adsorption prevented the soap fibers from restoring. The mechanical stability performance of grease was hence highly affected by the ZDDP and stearic acid.
3.3. Mechanical stability
The mechanical stability was investigated by performing traditional penetration measurements before and after the standardized roll stability test. Lubricating grease is usually considered stable to the continuous shear of rolling elements when its variation of penetration before and after the test is around zero.29 Table 2 shows the penetration values obtained from the pre-tested and post-tested lubricating greases using the roll stability tester. As previously analyzed, compared to the greases with ZDDP, greases without ZDDP had a more compact fiber structure (Fig. 2 and 3) and hence held relatively larger amounts of base fluid in the soap matrix. As a result, Table 2 shows that an increase of ZDDP concentration yielded an increase of penetration value and led to a decrease in the consistency. On the other hand, compared to grease A, whether there was ZDDP in grease B or not, the fiber structure was relatively looser. This was probably due to the decrease in the solubility of the soap by the stearic acid previously mentioned. The pre-tested penetration value of grease B was larger than that of grease A (Table 2). These results confirmed that the stearic acid led to the ideal fiber structure of the gelling network, while ZDDP destroyed the fiber structure.| ZDDP concentration (% (w/w)) | Grease A pre-tested penetration (dmm) | Grease B pre-tested penetration (dmm) | Grease A penetration after roll stability testing (dmm) | Grease B penetration after roll stability testing (dmm) | Grease A penetration change (dmm) | Grease B penetration change (dmm) |
|---|---|---|---|---|---|---|
| 0 | 258.75 ± 1.21 | 270.25 ± 0.41 | 269.25 ± 0.91 | 292.75 ± 1.01 | −10.5 ± 0.31 | −22.5 ± 0.51 |
| 1 | 260.25 ± 1.53 | 286.5 ± 0.73 | 272.5 ± 0.66 | 324.5 ± 1.32 | −12.25 ± 0.52 | −28 ± 0.32 |
| 2 | 264 ± 1.20 | 295.25 ± 1.25 | 276.25 ± 1.11 | 327.75 ± 0.95 | −12.25 ± 0.81 | −32.5 ± 0.41 |
| 3 | 270.25 ± 1.41 | 298.75 ± 1.11 | 283.75 ± 0.83 | 332.5 ± 0.71 | −13.5 ± 0.41 | −33.75 ± 0.73 |
| 4 | 279 ± 0.83 | 299.75 ± 0.72 | 295.5 ± 1.17 | 336 ± 0.91 | −16.5 ± 0.67 | −36.25 ± 0.85 |
| 5 | 280.75 ± 0.61 | 306.75 ± 0.43 | 300.5 ± 1.22 | 350 ± 0.73 | −19.75 ± 083 | −43.25 ± 0.64 |
| 6 | 296.5 ± 0.53 | 308.75 ± 1.25 | 334.25 ± 0.81 | 357.75 ± 1.12 | −37.5 ± 0.85 | −49 ± 0.91 |
| 7 | 299.5 ± 1.12 | 311.75 ± 0.91 | 339.75 ± 0.75 | 373 ± 1.2 | −40.25 ± 0.72 | −61.25 ± 1.13 |
The shear on the samples used on the roll stability tester at 75 °C was much stronger than that on the rheometer. The post-tested greases shown in Fig. 2e–h were composed of small and short fibers in a loosely tangled network, which indicated the destruction of fibers by the shear stress after the roll stability test. Thereby, the shear on the samples broke not only the weak bonds of cross-linked aggregates but also the thickener fibers, and eventually a certain degree of non-reversible fibrous structural breakdown occurred. Moreover, ZDDP was subsequently adsorbed on the new fibers' surfaces and prevented them from restoring. In contrast, due to the modification of surplus stearic acid, grease A was not so easily destroyed as grease B. Thus, grease B with ZDDP had shorter fibers than the others after the roll stability test. Compared with short fibers, the long fibers have more surface contact with the neighboring fibers and are able to resist deformation. In Table 2, the higher the ZDDP concentration was, the larger the negative increment of the penetration values would be, which was more significant for the case of grease B. The influence of ZDDP on the mechanical stability of grease B was larger than on that of grease A. Furthermore, similar to the plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G0N, there was a dramatic increase of the negative increment of the penetration values with ZDDP content after the roll-stability test, which was at the critical concentration of around 5 wt% for grease A and 4 wt% for grease B (Table 2). A much poorer mechanical stability of the lubricating grease, with the ZDDP concentration above 5 wt% for grease A and 4 wt% for grease B, was thus deduced, and this critical concentration could be considered as the maximum recommended concentration in the formulation of lubricating greases.
G0N, there was a dramatic increase of the negative increment of the penetration values with ZDDP content after the roll-stability test, which was at the critical concentration of around 5 wt% for grease A and 4 wt% for grease B (Table 2). A much poorer mechanical stability of the lubricating grease, with the ZDDP concentration above 5 wt% for grease A and 4 wt% for grease B, was thus deduced, and this critical concentration could be considered as the maximum recommended concentration in the formulation of lubricating greases.
4. Conclusions
Systematic studies of the influence of ZDDP concentrations on the fiber structure as well as on the rheological and mechanical properties of LCBG were conducted, and the following conclusions are drawn.1. Loose fiber with a large and sparse structure has been clearly observed for grease with ZDDP, while by adding trace amounts of surplus stearic acid, more compact fibers in the form of long, twisted ribbons were found for grease without ZDDP. Surplus stearic acid makes the structure of the soap fibers optimal in terms of yielding a stronger structure, a higher density of entanglements and reduction in solvency between the oil and thickener.
2. The shear stress, the linear viscoelasticity functions and mechanical stability decrease with the increase of ZDDP concentration. The influence of ZDDP concentration on the rheological and mechanical stability of low-alkali grease is greater than on those of low-acid grease.
3. Two different regions in the plateau modulus versus ZDDP concentration plot have been noticed, from which a much higher ZDDP concentration susceptibility of the lubricating grease at concentration above 4 wt% for low-alkali grease and 5 wt% for low-acid grease was obviously noticed. This critical concentration could be considered as the maximum recommended concentration in the formulation of lubricating greases, related to the strength of the microstructural network and mechanical stability of the grease affected by ZDDP concentration.
4. The saturated adsorption of ZDDP on the soap fibers as the concentration rose above 3 wt% makes it sufficiently migrate to the polar metallic surface and dampens the phenomenon of wall slip, which may enhance the anti-wear of grease. The 3 wt% addition of ZDDP is experimentally approved to be technologically desirable.
5. Owing to the fibrous instabilities of the thickener above 60 °C, a transformation from shear shinning to shear thickening has been found at the shear rate of 1800 s−1 and 80 °C. The grease microstructure was sufficiently destroyed, which led the fibers to loosen or even rupture. A considerable shortening of the thickener's fiber and increases in the penetration values were observed after the 24 hour long shearing of the grease in a roll stability tester at 75 °C. Due to the adsorption of ZDDP and stearic acid onto the new surface amongst the ruptured soap fibers, the turning of shear thickening is depressed. But the influences of ZDDP and stearic acid on the mechanical stability of grease are very different.
Despite findings shown in this work, further investigations should be carried out to develop theory-based models related to grease properties and the lubrication conditions (i.e. the elastohydrodynamic lubrication film formation).
References
- L. Jan and H. Erik, Tribol. Int., 2000, 33, 217–223 CrossRef
.
- M. Paszkowski and S. Olsztyńska-Janus, Ind. Lubr. Tribol., 2014, 2, 223–237 CrossRef
.
- R. Mas and A. Magnin, J. Rheol., 1994, 38, 889–908 CrossRef CAS
.
- H. A. Barnes, J. Non-Newtonian Fluid Mech., 1997, 70, 1–33 CrossRef CAS
.
- H. A. Barnes, J. Rheol., 1989, 33, 29–33 Search PubMed
.
- M. Paszkowski, in Tribology-fundamentals and advancements, ed. J. Gegner, InTech, Rijeka, 2013, pp. 77–106 Search PubMed
.
- J. B. Pan, Y. H. Cheng and J. Y. Yang, RSC Adv., 2015, 2015(5), 58686–65869 Search PubMed
.
- M. A. Nicholls, T. Do and P. R. Norton, Tribol. Int., 2005, 38, 15–39 CrossRef CAS
.
- H. Spikes, Tribol. Lett., 2004, 17, 469–489 CrossRef CAS
.
- T. J. Shen, Q. L. Liu and C. X. Lu, Lubr. Eng., 2006, 177, 121–124 Search PubMed
.
- M. R. Sivik, J. B. Zeitz and D. Bayus, NLGI Spokesman, 2001, 66, 20–24 Search PubMed
.
- Y. Shan, T. J. Shen and Z. Zhen, Lubr. Eng., 2007, 32, 102–105 CAS
.
- B. D. Mittal, E. Sayanna and K. P. Naithani, Lubr. Sci., 1998, 10, 171–176 CrossRef CAS
.
- P. Whittingstall, NLGI Spokesman, 1997, 61, 12–23 CAS
.
- S. Nolan, ELGI January/February, 2004, pp. 31–40 Search PubMed
.
- S. J. Nolan and M. R. Sivik, NLGI Spokesman, 2005, 69, 14–23 CAS
.
- J. L. Mansot, P. Terech and M. Martin, Colloids Surf., 1989, 39, 321–333 CrossRef CAS
.
- S. K. Yeong, P. F. Luckham and T. F. Tadros, J. Colloid Interface Sci., 2004, 274, 285–293 CrossRef CAS PubMed
.
- M. A. Delgado and C. Valencia, Ind. Eng. Chem. Res., 2006, 45, 1902–1910 CrossRef CAS
.
- S. Linda and S. Gunnar, Tribol. Trans., 2007, 63, 38–46 Search PubMed
.
- J. M. Franco, M. A. Delgado, C. Valencia, M. C. Sánchez and C. Gallegos, Chem. Eng. Sci., 2005, 60, 2409–2418 CrossRef CAS
.
- M. A. Delgado, J. M. Franco, C. Valencia, M. C. Sánchez and C. Gallegos, Chem. Eng. Res. Des., 2005, 83, 1085–1092 CrossRef CAS
.
- A. Adhvaryu, C. Sung and S. Z. Erhan, Ind. Crops Prod., 2005, 21, 285–291 CrossRef CAS
.
- T. J. Shen, M. H. Hu, R. G. Liu and Q. L. Liu, Tribology, 2011, 31, 581–586 CAS
.
- L. J. Clarke and P. J. Shuff, Tribol. Int., 1991, 24, 381–387 CrossRef
.
- G. V. Browning, NLGI Spokesman, 1950, 14, 10–15 CAS
.
- M. Paszkowski, Colloids Surf., A, 2015, 480, 462–467 CrossRef CAS
.
- M. E. Hunter and R. F. Baker, NLGI Spokesman, 2000, 63, 14–21 CAS
.
- J. E. Martín-Alfonso, G. Moreno and C. Valencia, J. Ind. Eng. Chem., 2009, 15, 687–693 CrossRef
.
- M. Paszkowski, Proc. Inst. Mech. Eng., Part J, 2013, 3, 209–219 CrossRef
.
- Y. G. Meng and J. Zheng, Tribol. Int., 1998, 31, 619–625 CrossRef CAS
.
- T. F. Tadros, Rheology of dispersions: Principles and applications, Wiley-VCH Publishers, Printed in Singapore, 2010, pp. 49–50 Search PubMed
.
- M. A. Delgado, C. Valencia, M. C. Sánchez and J. M. Franco, Tribol. Lett., 2006, 23, 47–54 CrossRef CAS
.
- T. J. Shen, C. X. Lu and Q. L. Liu, Acta Pet. Sin., 2009, 25, 15–21 CAS
.
- C. Balan and J. M. Franco, Tribol. Trans., 2001, 44, 53–58 CrossRef CAS
.
- M. Paszkowski, S. Olsztyńska-Janus and I. Wilk, Tribol. Lett., 2014, 56, 107–117 CrossRef CAS
.
- P. Coussot, A. L. Leonov and J. M. Piau, J. Non-Newtonian Fluid Mech., 1994, 44, 179–217 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2016 |