SiO2–carbon nanocomposite anodes with a 3D interconnected network and porous structure from bamboo leaves
Abstract
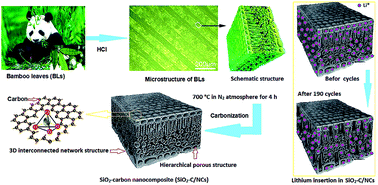
To seek for a low-cost, green and sustainable method of preparing nanostructured carbon electrode materials, we are inspired by natural biomaterials. An amorphous SiO2–carbon nanocomposite (SiO2–C/NCs) with three-dimensional (3D) interconnected network and hierarchical porous structure is synthesized by thermal decomposition of abandoned bamboo leaves at 700 °C in N2 atmosphere. The characterization results indicate that the SiO2–C/NCs inherited the natural hierarchical structure of the bamboo leaves. Compared with the commercialized graphite anode and other artificial nanostructured carbon materials, the SiO2–C/NCs anode shows a high lithium-storage capacity of 586.2 mA h g−1 at 200 mA g−1, with impressive good cycle stability (294.7 mA h g−1 after 190 cycles) and ultra-high coulombic efficiency close to 100%. After 160 cycles at varied current densities from 200 mA g−1 to 2000 mA g−1, this anode still maintains a high discharge of 117.4 mA h g−1. This simple, green and sustainable strategy will open a new avenue for large-scale preparation and application of nanostructured electrode materials from biomass materials.

 Please wait while we load your content...
Please wait while we load your content...