Mesoporous Au nanotube-constructed three-dimensional films with excellent SERS performance based on the nanofiber template-displacement reaction strategy†
Abstract
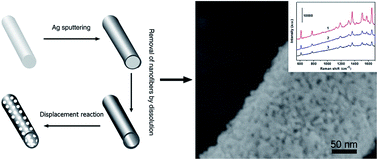
A facile and high-throughput strategy is presented to fabricate three-dimensional hierarchically porous Au films with good flexibility and transferability via displacement reaction of a Ag-coated electrospun nanofiber template. The films are constructed by mesoporous Au nanotubes, homogeneous in the macroscale but rough and porous in the nanoscale. Each nanotube-block is micro/nanostructured with evenly distributed mesopores on the tubewalls. Further experiments have revealed that the Ag sputtering time and displacement reaction conditions are the key influential factors determining the film architecture. Such structured film has exhibited significant surface-enhanced Raman scattering activity with good stability and reproducibility and shown the possibility of molecule-level detection. Additionally, the strategy is general for fabricating other hierarchically porous Au films, such as mesoporous Au hollow sphere arrays.

 Please wait while we load your content...
Please wait while we load your content...