BiOBr/Bi2MoO6 composite in flower-like microspheres with enhanced photocatalytic activity under visible-light irradiation†
Yingchun Miaoab,
Haibo Yina,
Lu Penga,
Yuning Huo*a and
Hexing Li*a
aThe Education Ministry Key Lab of Resource Chemistry, Shanghai Key Laboratory of RE Functional Materials, Shanghai Normal University, Shanghai 200234, China. E-mail: Hexing-li@shnu.edu.cn; huoyuning@shnu.edu.cn; Fax: +86-21-6432-2272; Tel: +86-21-6432-1673
bFaculty of Chemical and Engineering, Key Laboratory of Environment Chemistry, Qujing Normal University, Qujing 655000, China
First published on 15th January 2016
Abstract
BiOBr/Bi2MoO6 composites in flower-like microspheres were synthesized by a facile solvothermal method. This photocatalyst could be activated by visible light owing to the narrow energy band gaps of both Bi2MoO6 and BiOBr. The BiOBr/Bi2MoO6 composites exhibited high activity in the photocatalytic degradation of rhodamine B and other organic pollutants under visible-light irradiation owing to enhanced light harvesting via multiple reflections inside the flower-like structure and due to the reduced photoelectron–hole recombination due to the formation of BiOBr–Bi2MoO6 heterojunctions. The dominant active species during the photocatalytic oxidation of RhB were determined as photogenerated holes and ·O2− was distinguished as the sub-main active site. The RhB mineralized into CO2 through the de-ethylation route via forming five intermediates.
Introduction
Bismuth-containing semiconductors represent a new class of promising photocatalysts useful for environmental cleaning and H2 production, owing to their narrow energy band gaps for absorbing visible light, and their hierarchical structure and morphology as well as excellent stability.1,2 γ-Bi2MoO6 has received increasing interest due to its layered Aurivillius structure, in which perovskite slabs of corner-sharing distorted MoO6 octahedra are sandwiched between (Bi2O2)2+ layers,3,4 leading to the fast separation of photo-induced electrons from holes and thus, a high quantum efficiency of photocatalysis.5,6 To further improve its photocatalytic activity, both nanostructure engineering and composition doping have been explored.7 The combination of two semiconductors allows the transfer of photo-induced electrons from the valence band (VB) of one semiconductor to the conduction band (CB) of the other semiconductor, which could efficiently inhibit photoelectron–hole recombination. Up to now, Bi2MoO6-based composites with Bi2O3,8 ZnTiO3,9 Bi2WO6,10 TiO2,11 Bi2O2CO3,12 graphene13 and C60 (ref. 14) have been reported. However, their photocatalytic activities are still limited by the not well-matched band potentials to a certain extent.5,6BiOBr crystal with a tetragonal matlockite structure is composed of [Bi2O2]2+ slabs interleaved by double slabs of Br atoms. The layered BiOBr structure endows high carrier mobility and small probability of the recombination of photogenerated electrons and holes.15,16 We found that well-matched band potentials in BiOBr/Bi2MoO6 heterojunctions could be obtained, since the wider Eg and low CB potential in BiOBr compared to those in Bi2MoO6 could facilitate the transfer of photogenerated electrons from Bi2MoO6 to the CB of BiOBr, leading to efficient charge separation and high photocatalytic activity.
Herein, we fabricated BiOBr/Bi2MoO6 flower-like microspheres with a uniform BiOBr/Bi2MoO6 combination by a facile solvothermal route. The microspheres exhibited high activity in the photocatalytic degradation of organic pollutants under visible-light irradiation owing to the narrow energy band gap and unique morphology and due to the BiOBr–Bi2MoO6 heterojunctions. Meanwhile, the BiOBr/Bi2MoO6 combination displayed an unusual photocatalysis process.
Results and discussion
Structure characteristics
EDX analysis (Fig. S1†) demonstrated that only Bi, O, Br and Mo existed in a 0.20-BiOBr/Bi2MoO6 sample. As shown in Table 1, the ICP analysis revealed that the BiOBr content increased gradually in X-BiOBr/Bi2MoO6 with the increase of X. From Fig. 1, the XRD patterns of X-BiOBr/Bi2MoO6 samples (X < 1.5) only show the diffraction peaks indicative of rhombohedral Bi2MoO6 (JSPDS 21-0102), possibly due to the low content and high dispersion of BiOBr. With the increase of BiOBr content, the diffraction peaks at (011), (012) and (212) characteristic of BiOBr (JCPDS 73-2061) appear in the X-BiOBr/Bi2MoO6 samples with X ≥ 1.5. No other diffraction peaks could be observed, implying the formation of a pure BiOBr/Bi2MoO6 composite.| Sample | ICP | XPS | SBET (m2 g−1) | RhB adsorption rate (%) | |||
|---|---|---|---|---|---|---|---|
| Sample mass (g) | Bi concentration (mg L−1) | Mo concentration (mg L−1) | Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Br Br![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo molar ratio Mo molar ratio |
Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Br Br![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo molar ratio Mo molar ratio |
|||
| Bi2MoO6 | — | — | — | — | — | 19 | 8.7 |
| 0.10-BiOBr/Bi2MoO6 | 0.021 | 29 | 64 | 2.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.077 0.077![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
3.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.26 0.26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
19 | 15 |
| 0.20-BiOBr/Bi2MoO6 | 0.026 | 35 | 76 | 2.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.14 0.14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.45 0.45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
19 | 17 |
| 0.33-BiOBr/Bi2MoO6 | 0.023 | 31 | 65 | 2.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.22 0.22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
3.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.64 0.64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
21 | 18 |
| 0.50-BiOBr/Bi2MoO6 | 0.024 | 33 | 64 | 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35 0.35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
4.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
29 | 21 |
| BiOBr | — | — | — | — | — | 35 | 23 |
The FT-IR spectra in Fig. S2† revealed that all the samples displayed the vibration peaks of the surface-adsorbed water and hydroxyl groups at 3456 and 1625 cm−1, respectively.17,18 Corresponding to the above XRD results, 0.20-BiOBr/Bi2MoO6 displayed the same absorbance peaks as the pure Bi2MoO6 rather than the pure BiOBr due to the low content of BiOBr in the composite. The absorbance signals around 445 and 560 cm−1 could be assigned to the vibrations from the bending Mo–O bond of MoO6 and the bending Bi–O bond of (Bi2O2)2+ in Bi2MoO6,19 respectively. Meanwhile, the vibration peaks at 842, 797 and 733 cm−1 could be attributed to the asymmetric stretching of MoO6.19 In comparison, 1.5-BiOBr/Bi2MoO6 showed a well-resolved peak at 509 cm−1, indicative of the vibration of Bi–O bond in BiOBr.20
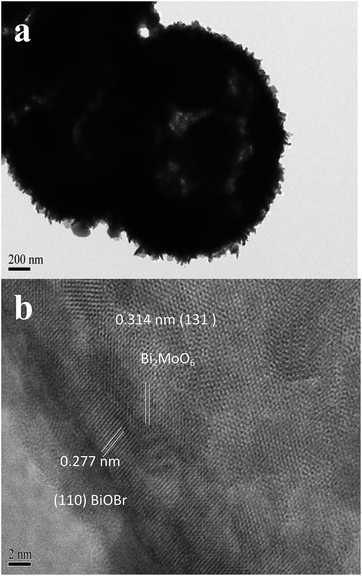
As shown in Fig. 2, the FESEM images demonstrated that 0.20-BiOBr/Bi2MoO6 was present in the uniform flower-like microspheres with the average diameter around 2.0 μm. The outer shell was covered by orderly assembled nanosheets with an average thickness of about 15 nm. The TEM image displayed a hollow chamber in the BiOBr/Bi2MoO6 microspheres, and the average shell thickness was estimated at 500 nm in Fig. 3. The HRTEM image clearly shows the lattice distances of 0.277 and 0.314 nm indicative of the (110) facet in the BiOBr crystal21 and the (131) facet in the Bi2MoO6 crystal,9 respectively, corresponding to the formation of BiOBr–Bi2MoO6 heterojunctions at the interface. Moreover, the chemical mapping (Fig. S3†) confirmed the uniform distribution of Bi, Mo, Br and O elements in the microspheres.
The XPS spectra in Fig. 4 confirmed that the pure Bi2MoO6 displayed a Bi binding energy around 158.7 and 164.0 eV in Bi 4f5/2 and 4f7/2 levels,22,23 while the pure BiOBr showed higher Bi 4f binding energies of around 159.4 and 164.7 eV.24,25 The Bi 4f binding energy of 0.20-BiOBr/Bi2MoO6 was between that of Bi2MoO6 and BiOBr, which implied an electronic interaction in the BiOBr/Bi2MoO6 composite. Additionally, 0.20-BiOBr/Bi2MoO6 displayed higher binding energies of both O 1s and Mo 3d compared to pure Bi2MoO6 as well as a lower binding energy of O 1s and Br 3d than that of pure BiOBr. This could be attributed to the electron transfer from Bi2MoO6 to BiOBr via the strong interaction in the BiOBr/Bi2MoO6 heterojunction, corresponding to the above HRTEM image. The higher molar ratio of both Bi/Mo and Br/Mo in the different X-BiOBr/Bi2MoO6 samples determined by XPS compared to those by ICP analysis (see Table 1) further suggested the surface enrichment of Bi and Br atoms, which was obviously due to the coverage of BiOBr on the surface of the Bi2MoO6 microspheres.
The TG analysis (Fig. S4†) revealed that pure Bi2MoO6 showed no significant weight loss, while the pure BiOBr displayed a significant weight loss around 510 °C due to the release of Br-contained gas via BiOBr oxidation.25 The BiOBr/Bi2MoO6 showed a higher weight loss temperature than the pure BiOBr. This further confirmed the strong BiOBr–Bi2MoO6 interaction, in which the Bi2MoO6 could stabilize the BiOBr.
All the BiOBr, Bi2MoO6 and X-BiOBr/Bi2MoO6 samples displayed typical IV N2 adsorption–desorption isotherms (Fig. S5†), indicating the presence of a mesoporous structure. As shown in Table 1, SBET of the X-BiOBr/Bi2MoO6 samples gradually increased with the enhanced content of BiOBr in the BiOBr/Bi2MoO6 composite since the SBET of pure BiOBr was higher than that of pure Bi2MoO6.
Optical properties
The UV-vis DRS spectra (Fig. S6†) revealed that pure Bi2MoO6 displayed stronger absorbance in the wider visible-light region compared to that of pure BiOBr due to its narrower energy band gap. The 0.20-BiOBr/Bi2MoO6 displayed slightly enhanced visible-light absorbance as the BiOBr nanosheets assembled on the outer shell of Bi2MoO6 microspheres could enhance light harvesting via the multi-reflections. Fig. S7a† shows that 0.20-BiOBr/Bi2MoO6 displays the highest photocurrent compared to pure BiOBr, pure Bi2MoO6 and a mechanical mixture of BiOBr and Bi2MoO6, which could be attributed to both the enhanced light harvesting and the diminished photoelectron–hole recombination rate. The PL spectra (Fig. S7b†) clearly confirmed a lower photoelectron–hole recombination rate in the X-BiOBr/Bi2MoO6 composites than in pure BiOBr, pure Bi2MoO6, and in a mechanical mixture of BiOBr and Bi2MoO6, corresponding to the weaker PL intensities.21,26 These results demonstrate that the formation of BiOBr–Bi2MoO6 heterojunction plays a key role in inhibiting photoelectron–hole recombination via transferring photoelectrons from the VB of Bi2MoO6 to the CB of BiOBr.27 The mechanical mixture of BiOBr and Bi2MoO6 exhibited a higher photoelectron–hole recombination rate than the BiOBr/Bi2MoO6 composites, obviously due to the poor interaction between BiOBr and Bi2MoO6, leading to fewer BiOBr–Bi2MoO6 heterojunctions. Besides, it was found that the photoelectron–hole recombination rate decreased with the increase of BiOBr from 0.10-BiOBr/Bi2MoO6 to 0.20-BiOBr/Bi2MoO6, corresponding to the increase in the number of BiOBr–Bi2MoO6 heterojunctions. However, a very high BiOBr content was harmful for inhibiting photoelectron–hole recombination (see 0.50-BiOBr/Bi2MoO6 and 0.33-BiOBr/Bi2MoO6), possibly due to the formation of BiOBr and Bi2MoO6 in separated phases.Additionally, the flat band potentials (Efb) of BiOBr and Bi2MoO6 were determined in EIS (see Fig. S8†). According to the intercept of the linear relationship between CSC−2 and E, the Efb (CSC−2 = 0) of BiOBr and Bi2MoO6 were calculated. As shown in Table 2, the conduction band edge (ECB) was 0.1 V more negative than the Efb28 in n-type BiOBr and Bi2MoO6 semiconductors owing to the positive line slope.29 The edge position of the valence band could also be determined by considering the energy band gap calculated from ahv = A(hv − Eg)n, where a, v, Eg and A refer to the absorption coefficient, the light frequency, the band gap and a constant, respectively. The values of n for Bi2MoO6 and BiOBr were 1/2 and 2, respectively.30,31 Therefore, the energy band structure in the BiOBr/Bi2MoO6 composite is proposed, see Scheme 1. Clearly, the band edges of BiOBr (EVB = 2.57 eV, ECB = −0.19 eV) and Bi2MoO6 (EVB = 2.04 eV, ECB = −0.55 eV) were well matched and thus, their heterojunctions were favourable for the rapid transfer of photoelectrons from the VB of Bi2MoO6 to the VB of BiOBr, which reduces the photoelectron–hole recombination rate.
| Sample | Efb (eV) | ECB (eV) | EVB (eV) | Eg (eV) |
|---|---|---|---|---|
| Bi2MoO6 | −0.450 | −0.550 | 2.04 | 2.59 |
| BiOBr | −0.0900 | −0.190 | 2.57 | 2.76 |
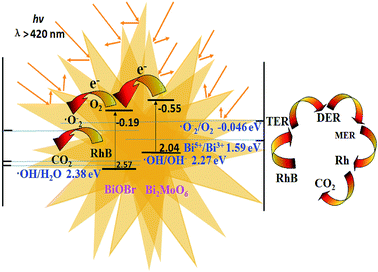 | ||
| Scheme 1 Illustration of the energy band structure and photocatalytic reaction process of the BiOBr/Bi2MoO6 heterojunction. | ||
Photocatalytic performance
As shown in Table 1, the BiOBr exhibited much higher adsorption capacity for RhB than the Bi2MoO6 owing to the larger SBET. It was interesting to note that the BiOBr exhibited much higher adsorption capacity per unit SBET than the Bi2MoO6, possibly due to the different adsorption models for RhB. Obviously, each BiOBr active site adsorbed more RhB molecules than each Bi2MoO6 active site, which could account for the higher adsorption capacity of the X-BiOBr/Bi2MoO6 than that of pure Bi2MoO6 with almost the same SBET.The visible-light-induced photocatalytic RhB degradation yields and TOC removal rates on different photocatalysts are summarized in Fig. 5. Although the BiOBr showed a much higher adsorption capacity than of the Bi2MoO6 (see Table 1), it still exhibited lower activity, which could be attributed to the broad energy band gap, corresponding to a poor utilization of visible light in comparison with the Bi2MoO6. The 0.20-BiOBr/Bi2MoO6 exhibited much higher activity than those of pure BiOBr, pure Bi2MoO6, the BiOBr + Bi2MoO6 mechanical mixture and 0.20-BiOBr/Bi2MoO6(G), in which 0.1 g of 0.20-BiOBr/Bi2MoO6 was ground for 120 min in the mortar, which damaged the flower-like structure of 0.20-BiOBr/Bi2MoO6 (see SEM images in Fig. S9†). Taking into account the similar adsorption capacities for RhB, the high activity of 0.20-BiOBr/Bi2MoO6 could be mainly attributed to both the enhanced light harvesting due to the multiple reflections in the unique flower-like structure and the diminished photoelectron–hole recombination rate owing to the formation of BiOBr–Bi2MoO6 heterojunctions resulting from their strong interaction.32–34 The low activities of pure BiOBr and Bi2MoO6 were due to the easy recombination of photo-induced holes and electrons, and the slightly increased activity of the BiOBr + Bi2MoO6 sample may be due to the random and unstable contact between Bi2MoO6 and BiOBr in the physical mixture, which is favourable for the charge separation to some extent. However, it is impossible to form stable BiOBr–Bi2MoO6 heterojunctions to greatly improve the photocatalytic activity. The 0.20-BiOBr/Bi2MoO6(G) sample exhibited lower activity than 0.20-BiOBr/Bi2MoO6 due to the damage to the flower-like structure (see Fig. S9†). The TOC measurement showed the change in the total amount of organics, including the original RhB and the intermediates during the photocatalytic process. Both the original RhB and the intermediates were photodegraded simultaneously. Therefore, the decreased TOC value was obviously lower than that of the RhB degradation ratio together with a certain difference in activity (Fig. 5b), and RhB was photodegraded to CO2. By plotting (C0 − C)/C vs. reaction time, linear plots were obtained for all of the prepared photocatalysts (see Fig. S10†), which indicated that the RhB degradation reaction was a zero-order reaction with respect to RhB concentration. 0.22-BiOBr/Bi2MoO6 showed the very highest kinetic constants due to the highest photocatalytic efficiency. Similar results were also obtained during visible-light-induced photocatalytic degradation of methyl orange (MO) and phenol (Fig. S11†). It was also found that in the X-BiOBr/Bi2MoO6 series, their activities first increased with the increasing BiOBr content from pure Bi2MoO6 to 0.10-BiOBr/Bi2MoO6 to 0.20-BiOBr/Bi2MoO6 and then decreased with the further increase of BiOBr content from 0.20-BiOBr/Bi2MoO6 to 0.33-BiOBr/Bi2MoO6 to 0.50-BiOBr/Bi2MoO6. The 0.20-BiOBr/Bi2MoO6 sample displayed the maximum activity (Fig. S12†). Such results were exactly the same as the changing order of PL intensities (Fig. S7†), suggesting that the photoelectron–hole recombination rate plays a crucial role in determining photocatalytic activity. The insufficient BiOBr–Bi2MoO6 heterojunctions in the 0.10-BiOBr/Bi2MoO6 sample resulted in an inefficient inhibition of charge recombination, while more BiOBr catalyst in both the 0.33-BiOBr/Bi2MoO6 and 0.50-BiOBr/Bi2MoO6 samples induced easier charge recombination.
In order to examine the formation of ˙OH radicals during the photodegradation reaction, a terephthalic acid photoluminescence (TA-PL) probing technique35,36 with an excitation wavelength of 315 nm was employed. In Fig. S13,† PL signals were observed at 425 nm with the irradiation time (excitation at 315 nm). This demonstrated that ˙OH radicals were formed during the photodegradation process. However, as shown in Fig. S13,† 0.20-BiOBr/Bi2MoO6 exhibited similar TA-PL peak intensity at 425 nm compared to Bi2MoO6, but much lower than BiOBr, implying that ˙OH radicals were mainly generated on BiOBr in the BiOBr/Bi2MoO6 composite. It could be ascribed that only the photo-induced holes on the BiOBr photocatalyst rather than that on Bi2MoO6 were able to oxidize either H2O or OH− directly to ˙OH, since both redox potentials of ˙OH/H2O (Eo = 2.38 V)37 and ˙OH/OH− (Eo = 2.27 V)38,39 were more positive than the EVB of Bi2MoO6, but were more negative than the EVB of BiOBr. Therefore, it was difficult to oxidize H2O or OH− into ˙OH in the Bi2MoO6/BiOBr heterojunction due to the low content of BiOBr. Additionally, Fig. 6 reveals that the addition of isopropanol (IPA) allowed it to act as an ˙OH scavenger,40,41 thus displaying no significant inhibiting effect on the photocatalytic RhB degradation. However, the addition of ammonium oxalate (AO) as h+ (photogenerated holes) scavengers42 could almost completely suppress the photocatalytic RhB degradation. The addition of benzoquinone (BQ) as an ˙O2− scavenger40,43 and AgNO3 as an e− scavenger44 also resulted in decreased activity, respectively. This demonstrated that h+ was the main active site and could oxidize the RhB directly rather than first following the process of forming ˙OH.
 | ||
| Fig. 6 Effects of various scavengers on the photocatalytic efficiencies of 0.20-BiOBr/Bi2MoO6 in RhB photocatalytic degradation. Reaction conditions are given in Fig. 5a. | ||
According to the abovementioned results, the plausible mechanism of RhB photocatalytic degradation on the BiOBr/Bi2MoO6 composite photocatalyst is proposed in Scheme 1. Under visible-light irradiation, the electrons could be excited from the VB of Bi2MoO6 and BiOBr to their CB. The electrons in the CB of Bi2MoO6 were injected into the CB of BiOBr via their heterojunctions, followed by reacting with the adsorbed O2 to produce ˙O2−, owing to the higher redox potential of O2/˙O2− (Eo = −0.046 eV)45 than the ECB of BiOBr. Finally, ˙O2− and the main active species of h+ led to the oxidation of RhB.
In the photodecomposition process in dye wastewater treatment, it is important to evaluate the mineralization ability of catalysts since the yielded intermediates may be more toxic than the dye molecule itself. Since the multiple yielded intermediates may also exhibit activity on the visible-light-driven RhB degradation process,33 the photocatalytic RhB degradation yields and TOC removal rates under visible lights show certain difference of activity in Fig. 5a and b. Hence, the yielded intermediates of the visible-light-driven RhB degradation process on 0.20-BiOBr/Bi2MoO6 were further investigated by HPLC-MS (Fig. S14†). Moreover, the N,N,N′,N'-tetraethylrhodamine (RhB) molecule with an m/z of 443, five intermediates with m/z of 415, 387a, 387b, 359 and 331 were detected, corresponding to N,N,N′-triethylrhodamine (TER), N,N′-diethylrhodamine (DERa), N,N-diethylrhodamine (DERb), N-ethylrhodamine (MER) and rhodamine. The intermediates were not detected, as they were more toxic than the dye molecule itself. The RhB concentration decreased monologically with the increasing reaction time, while the concentrations of all the intermediates first increased and then decreased. According to the appearance order of multiple intermediates, we concluded that the RhB degradation followed the de-ethylation route and cleaved into TER, DER, MER and rhodamine in turn, and finally decomposed into CO2.
Conclusions
This work developed a facile solvothermal approach to prepare a BiOBr/Bi2MoO6 composite photocatalyst in flower-like microspheres. It exhibited high activity in the photocatalytic degradation of organic pollutants under visible-light irradiation, mainly owing to enhanced light harvesting via multi-reflections and reduced photoelectron–hole recombination through the heterojunction. Kinetic studies demonstrated that the RhB degradation to CO2 were mainly driven by h+ active sites and followed a de-ethylation route with the formation of five intermediates.Experimental section
BiOBr/Bi2MoO6 preparation
3.4 g Bi(NO3)3·5H2O and 0.84 g Na2MoO4·2H2O were dissolved in 10 mL ethylene glycol (EG) and 10 mL glycerol under stirring for 120 min, respectively. After mixing the two solutions, 40 mL ethanol was slowly added, followed by stirring for 10 min. The clear solution was then transferred into a 100 mL Teflon-lined autoclave and heated at 160 °C for 24 h. The brown suspension with Bi2MoO6 precursor was obtained after the autoclave was cooled to room temperature naturally. Then, the desired amounts of Bi(NO3)3·5H2O and cetyltrimethyl ammonium bromide (CTAB) with a 1.5 molar ratio to Bi(NO3)3·5H2O was added with stirring for 120 min. The solution was transferred into a 100 mL Teflon-lined autoclave and heated at 160 °C for 10 h again. Finally, the obtained solid was filtered, washed with ethanol, dried at 80 °C in air and annealed at 400 °C for 4 h. The as-prepared sample was denoted as X-BiOBr/Bi2MoO6, where X refers to the Br/Mo molar ratio. For comparison, the pure Bi2MoO6 and BiOBr samples were prepared by the same procedures, respectively. The mechanical mixture of BiOBr and Bi2MoO6 was referred to BiOBr + Bi2MoO6, in which the Br/Mo molar ratio was the same as that in 0.20-BiOBr/Bi2MoO6. The 0.20-BiOBr/Bi2MoO6(G) sample was 0.20-BiOBr/Bi2MoO6 after being crushed.Catalyst characterization
The structure and composition were determined by energy dispersive X-ray spectroscopy (EDX, HITACHI S-4800) and X-ray diffraction (XRD, Rigacu D/Max-2000). Surface morphologies were observed by scanning electron microscopy (FESEM, HITACHI S-4800) and by using a transmission electronic micrograph (TEM, JEM-2010). The thermal stability was investigated by thermogravimetric analysis (TGA) on a DTG-60H. The FTIR spectra were recorded by a Fourier transform infrared spectroscopy (NEXUS 470). X-ray photoelectron spectroscopy (XPS, Versa Probe PHI 5000) was employed to determine the surface electronic states. The shift of the binding energy due to the relative surface charging was corrected using the C1S level at 284.6 eV as an internal standard. N2 adsorption/desorption isotherms were measured on a TriStar II 3020. The Brunauer–Emmett–Teller (BET) method was used to calculate the specific surface area (SBET) based on the adsorption branches. The optical properties were determined by UV-vis diffuse reflectance spectrum (UV-vis DRS, Varian Cary 500) and photoluminescence spectrum (PL, Varian Cary-Eclipse 500). The excitation wavelength was 325 nm provided by a UV xenon lamp with an incidence angle of 45°, and the spectra were recorded in the range from 400 to 600 nm at room temperature. To avoid reflecting the excitation light into the detector, all the samples were maintained at 60° to the detection plane, which was defined by the Xe lamp, the samples and the detector. Meanwhile, ˙OH-trapping photoluminescence spectra of terephthalic acid solution (PL-TA, λexcition = 315 nm, λemission = 425 nm) were recorded on a photoluminescence spectrum (PL, Varian Cary-Eclipse 500). The metal ion concentrations were determined by using inductively coupled plasmatomic emission spectroscopy (ICP, VISTA-MPX). Photocurrent-time measurements and electrochemical impedance spectroscopy (EIS) were performed using an electrochemistry workstation (CHI660E). Photocurrent-time measurements were carried out on the film sample in 0.10 M Na2SO4 aqueous solution with an exposed surface area of 2.0 cm2, which was irradiated by a 300 W Xe lamp (λ > 420 nm). EIS Experiments were carried out on the film sample in 0.10 M Na2SO4 solution at 1000 Hz within the potential region of −1.0 to 1.0 V vs. Ag/AgCl. A Mott–Schottky (M–S) plot showed the linear variation of CSC2− with respect to the applied potential (eqn (1)), where ND, ε, Efb, E and CSC refer to the doping density, dielectric constant, flat band potential, external biasing voltage and capacitance, respectively.| CSC−2 = (1.41 × 1020/NDε)[E − Efb − 0.026] | (1) |
Activity test
The liquid-phase photocatalytic degradation of dyes was carried out at 30 °C in a self-designed 250 mL reactor containing 0.10 g catalyst and 100 mL 20 mg L−1 dye organics. A 300 W Xe lamp was used as a visible-light source, and a 420 nm filter was placed above the reactor to cut off the UV light. At given time intervals, 3.0 mL aliquots were collected from the suspension and immediately centrifuged. The concentration of dyes was determined using a UV-vis spectrophotometer (UV-7504 PC) at the characteristic peak. A liquid chromatography-mass spectrometry system (HPLC-MS, Agilent 1200) was used to detect the reaction products and intermediates. The measurement of total organic carbon (TOC) was carried out on a Vario TOC analyzer. The reproducibility of the results was checked by repeating the experiments at least three times and was found to be within acceptable limits (±6%).Acknowledgements
This work is supported by National Natural Science Foundation of China (21237003 and 21577092), Program for Changjiang Scholars and Innovative Research Team in University (IRT1269), Shanghai Government (15520711300) International Joint Laboratory on Resource Chemistry (IJLRC), Science and technology project of Yunnan Province (C0120150543) and Research Fund of Yunnan Provincial Education Department (2015Z183).Notes and references
- W. Z. Yin, W. Z. Wang and S. M. Sun, Catal. Commun., 2010, 11, 647–650 CrossRef CAS.
- A. Martinez-de Cruz and S. Obregon Alfaro, J. Mol. Catal. A: Chem., 2010, 320, 85–91 CrossRef.
- E. L. Cuéllar, A. M. Cruz, K. H. L. Rodríguez and U. O. Méndez, Catal. Today, 2011, 166, 140–145 CrossRef.
- L. W. Zhang, T. G. Xu, X. Zhao and Y. F. Zhu, Appl. Catal., B, 2010, 98, 138–146 CrossRef CAS.
- X. Lin, X. Y. Guo, W. L. Shi, H. J. Zhai, Y. S. Yan and Q. W. Wang, J. Solid State Chem., 2015, 229, 68–77 CrossRef CAS.
- J. Di, J. X. Xia, M. X. Ji, H. P. Li, H. Xu, H. M. Li and R. Chen, Nanoscale, 2015, 7, 11433–11443 RSC.
- J. Ren, W. Z. Wang, M. Shang, S. M. Sun and E. P. Gao, ACS Appl. Mater. Interfaces, 2011, 3, 2529–2533 CAS.
- Y. S. Xu, Z. J. Zhang and W. D. Zhang, Mater. Res. Bull., 2013, 48, 1420–1427 CrossRef CAS.
- P. Zhang, C. L. Shao, M. Y. Zhang, Z. C. Guo, J. B. Mu, Z. Y. Zhang, X. Zhang and Y. C. Liu, J. Hazard. Mater., 2012, 217–218, 422–428 CrossRef CAS PubMed.
- F. J. Zhang, S. F. Zhu, F. Z. Xie, J. Zhang and Z. D. Meng, Sep. Purif. Technol., 2013, 113, 1–8 CrossRef CAS.
- N. Li, L. Zhu, W. D. Zhang, Y. X. Yu, W. H. Zhang and M. F. Hou, J. Alloys Compd., 2011, 509, 9770–9775 CrossRef CAS.
- Y. S. Xu and W. D. Zhang, Appl. Catal., B, 2013, 140–141, 306–316 CrossRef CAS.
- F. Zhou, R. Shi and Y. F. Zhu, J. Mol. Catal. A: Chem., 2011, 340, 77–82 CrossRef CAS.
- X. Zhao, H. J. Liu, Y. L. Shen and J. H. Qu, Appl. Catal., B, 2011, 1–2, 63–68 Search PubMed.
- H. Deng, J. W. Wang, Q. Peng, X. Wang and Y. D. Li, Chem.–Eur. J., 2005, 11, 6519–6524 CrossRef CAS PubMed.
- W. L. Huang and Q. S. Zhu, Comput. Mater. Sci., 2008, 43, 1101–1108 CrossRef CAS.
- J. C. Yu, L. Z. Zhang, Z. Zheng and J. Zhao, Chem. Mater., 2003, 15, 2280–2286 CrossRef CAS.
- T. Y. Peng, D. Zhao, H. B. Song and C. H. Yan, J. Mol. Catal. A: Chem., 2005, 238, 119–126 CrossRef CAS.
- M. Maczka, L. Macalik, K. Hermanowicz, L. Kepinski and J. Hanuza, J. Raman Spectrosc., 2010, 41, 1289–1296 CrossRef CAS.
- Z. S. Liu, Y. H. Bi, Y. L. Zhao, X. Huang and Y. B. Zhu, Mater. Res. Bull., 2014, 49, 167–171 CrossRef CAS.
- D. Q. Zhang, M. C. Wen, B. Jiang, G. S. Li and J. C. Yu, J. Hazard. Mater., 2012, 211–212, 104–111 CrossRef CAS PubMed.
- X. Zhao, J. H. Qu, H. J. Liu and C. Hu, Environ. Sci. Technol., 2007, 41, 6802–6807 CrossRef CAS PubMed.
- G. P. Dai, J. G. Yu and G. Liu, J. Phys. Chem. C, 2011, 115, 7339–7346 CAS.
- X. M. Tu, S. L. Luo, G. X. Chen and J. H. Li, Chem.–Eur. J., 2012, 18, 14359–14366 CrossRef CAS PubMed.
- Y. C. Feng, L. Li, J. W. Li, J. F. Wang and L. Liu, J. Hazard. Mater., 2011, 92, 538–544 CrossRef PubMed.
- J. C. Yu, J. G. Yu, W. K. Ho, Z. T. Jiang and L. Z. Zhang, Chem. Mater., 2002, 14, 3808–3816 CrossRef CAS.
- J. Zhang, J. X. Xia, S. Yin, H. M. Li, H. Xu, M. Q. He, L. Y. Huang and Q. Zhang, Colloids Surf., A, 2013, 420, 89–95 CrossRef CAS.
- S. R. Morrison, Electrochemistry at Semiconductor and Oxidized Metal Electrodes, Plenum Press, New York, 1980, ch. 4 Search PubMed.
- J. M. Herrmann, M. E. Jamal and M. Forissier, React. Kinet. Catal. Lett., 1988, 37, 255–260 CrossRef CAS.
- M. Y. Zhang, C. L. Shao, P. Zhang, C. Y. Su, X. Zhang and P. P. Liang, J. Hazard. Mater., 2012, 225–226, 155–163 CrossRef CAS PubMed.
- X. J. Shi, X. Chen, X. L. Chen, S. M. Zhou, S. Y. Lou, Y. Q. Wang and L. Yuan, Chem. Eng. J., 2013, 222, 120–127 CrossRef CAS.
- J. Cao, B. Y. Xu, H. L. Lin, B. D. Luo and S. F. Chen, Chem. Eng. J., 2012, 185–186, 91–99 CrossRef CAS.
- Y. C. Miao, G. F. Pan, Y. N. Huo and H. X. Li, Dyes Pigm., 2013, 99, 382–389 CrossRef CAS.
- H. X. Li, Z. F. Bian, J. Zhu, D. Q. Zhang, G. S. Li, Y. N. Huo, H. Li and Y. F. Lu, J. Am. Chem. Soc., 2007, 129, 8406–8407 CrossRef CAS PubMed.
- K. Ishibashi, A. Fujishima, T. Watanabe and K. Hashimoto, Electrochem. Commun., 2000, 2, 207–210 CrossRef CAS.
- Q. Xiao, Z. C. Si, J. Zhang, C. Xiao and X. K. Tan, J. Hazard. Mater., 2008, 150, 62–67 CrossRef CAS PubMed.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods: Fundamentals and Applications, John Wiley & Sons, New York, 1980 Search PubMed.
- P. L. Allen and A. Hickling, Trans. Faraday Soc., 1967, 53, 1626–1635 RSC.
- X. Li, J. Zhu and H. X. Li, Appl. Catal., B, 2012, 123–124, 174–181 CrossRef CAS.
- G. T. Li, K. H. Wong, X. W. Zhang, C. Hu, J. C. Yu, R. C. Y. Chan and P. K. Wong, Chemosphere, 2009, 76, 1185–1191 CrossRef CAS PubMed.
- L. S. Zhang, K. H. Wong, H. Y. Yip, C. Hu, J. C. Yu, C. Y. Chan and P. K. Wong, Environ. Sci. Technol., 2010, 44, 1392–1398 CrossRef CAS PubMed.
- N. Zhang, S. Q. Liu, X. Z. Fu and Y. J. Xu, J. Phys. Chem. C, 2011, 115, 9136–9145 CAS.
- M. C. Yin, Z. S. Li, J. H. Kou and Z. G. Zou, Environ. Sci. Technol., 2009, 43, 8361–8366 CrossRef CAS PubMed.
- Y. N. Huo, J. Zhang, M. Miao and Y. Jin, Appl. Catal., B, 2012, 111–112, 334–341 CrossRef CAS.
- L. Kong, Z. Jiang, H. H. Lai, R. J. Nicholls, T. C. Xiao, M. O. Jones and P. P. Edwards, J. Catal., 2012, 293, 116–125 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra18987j |
| This journal is © The Royal Society of Chemistry 2016 |