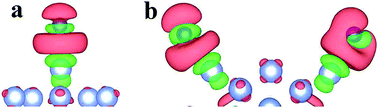Highly sensitive and selective colorimetric detection of sulphide using Ag–Au nanoalloys: a DFT study
Le Changa,
Adrian Fisherb,
Zhiping Liu*a and
Daojian Cheng*abc
aState Key Laboratory of Organic-Inorganic Composites, Beijing University of Chemical Technology, Beijing 100029, People's Republic of China. E-mail: chengdj@mail.buct.edu.cn; liuzhp@mail.buct.edu.cn; Fax: +86-10-64427616
bInternational Research Center for Soft Matter, Beijing University of Chemical Technology, Beijing 100029, People's Republic of China
cChangzhou Institute of Advanced Materials, Beijing University of Chemical Technology, Changzhou 213164, People's Republic of China
First published on 4th February 2016
Abstract
A density functional theory approach is applied to investigate the sensing mechanism for the colorimetric detection of sulphide (S) among sulphide species, such as S, SH, cysteine (Cys), and H2S, using Ag–Au nanoalloys. By exploring the adsorption of sulphide species on the Ag42Au13 and Ag55 clusters, it is found that the adsorption strength of those sulphide species on the Ag42Au13 cluster is stronger than those on the Ag55 cluster, corresponding to the higher sensitivity of the Ag42Au13 cluster compared with the Ag55 one for the colorimetric detection of sulphide species. In addition, it is found that the adsorption strength of the Ag42Au13 and Ag55 clusters towards sulphide species follows the order of S > SH > Cys > H2S, indicating that both the Ag42Au13 and Ag55 clusters possess high selectivity for the colorimetric detection of S over other sulphide species. By investigating the coverage effect of S on the Ag42Au13 cluster, it is found that increasing the coverage of S leads to the decrease of the adsorption strength. Our theoretical results are expected to provide new guidelines for rational design of more powerful adsorption-based colorimetric sensors for detecting S using Ag–Au nanoalloys.
1. Introduction
Sulphide (S) is widely presented in cellular biological environments in the form of aqueous S, SH, cysteine (Cys), H2S and so on. Since these small molecules are active in live cells and mediate a wide range of physiological, pathological, anabolic and catabolic processes,1,2 it is crucial to understand their biological activities and functions.3 The key challenge is to provide attractive and promising solutions for tracking biologically active sulphide species among other biomolecules rapidly.4,5 In recent years, great advancements in selectively detecting S have been made, and current strategies mainly include fluorescence strategies6,7 and non-fluorescence strategies.8 Among non-fluorescence strategies, adsorption-based colorimetric strategy has been considered to be the most important method in detecting S,9,10 due to its performability and relatively low cost. For example, Deng and coworkers11 have achieved colorimetric recognition of S in S-containing proteins, which can be simply selectively detected with naked eyes. Montoya and coworkers9 have developed colorimetric probes for detecting S based on the different adsorption strength among various sulphide species, which is proved to be unaffected by the presence of other biologically relevant nucleophiles. However, new colorimetric sensors for detecting S still need to be developed to understand the activity of S in biological environments.More recently, Au and Ag nanoparticles (NPs) have received extensive attention in a wide variety of sensing researches12–14 and technical applications15–17 in biological environment due to its good selectivity18–20 for detecting S based on the formation of Au2S21 and Ag2S.22 For example, Zhang et al.23 utilized Au NPs to selectively detect sulphide species, where color changes from blue to red could be observed with naked-eye. Moreover, core–shell Ag–Au bimetallic clusters (or “nanoalloys”) have been used to detect S, showing excellent sensitivity and selectivity18–20 for detecting S.24 Xiong and coworkers25 have proved that core–shell Ag–Au nanoalloys exhibit high sensitivity and good selectivity for the colorimetric detection of S among S, SH, Cys, and H2S. However, the sensing mechanism for detecting S using Ag–Au nanoalloys over other sulphide species as colorimetric sensors is still unclear.
Computational methods have become an extremely powerful tool for providing information at the atomic and electronic levels. Depending on the adsorption strength, the selectivity of colorimetric sensors can be observed by density functional theory (DFT) calculations.15 For example, Haque and coworkers26 performed DFT calculations to verify the selectivity of the colorimetric sensor by distinguishing the adsorption strength. It was proved that better selectivity corresponds to stronger adsorption strength. In addition, Pang et al.27 found that the selectivity depends on the adsorption strength of colorimetric sensor towards small molecules. However, less theoretical work has been done to study the sensitivity and selectivity of Ag–Au nanoalloys as colorimetric sensors for detecting S by DFT calculations.
In this work, the colorimetric sensing properties of Ag–Au nanoalloys for detecting S among sulphide species, such as S, SH, Cys, and H2S, are investigated by DFT calculations. The adsorption strengths of S, SH, Cys, and H2S on the Ag42Au13 and Ag55 clusters are calculated to understand the sensitivity and selectivity of these clusters towards sulphide species. The detailed sensing mechanism for the colorimetric detection of S using the Ag42Au13 and Ag55 clusters over other sulphide species is explained by the density of states and charge differences of these clusters. In addition, the coverage effect of S on the Ag42Au13 cluster is investigated by analyzing the charge difference redistribution.
2. Methodology and calculation details
DFT calculations were performed with the spin-polarized plane-wave method implemented in the Quantum Espresso package.28 The Perdew–Burke–Ernzerhof (PBE) functional based on the generalized gradient approximation (GGA)29 were employed to evaluate the non-local exchange-correlation (xc) energy. In a 30 × 30 × 30 Å cubic supercell, the values of 40 and 400 Ry were used as the kinetic energy cutoff for wave functions and charge densities. The possible positions of the atoms in the complex were fully optimized until the forces were smaller than 0.01 eV Å−1 per atom. The first Brillouin zone was sampled at the Γ point, and the electronic levels were broadened with a Gaussian smearing of about 0.002 Ry.30 The self-consistent field calculation has convergence criteria of 10−6 Hartree.In this work, 55-atom Ag42Au13 and Ag55 clusters with Mackay icosahedral structure, which is commonly found as the lowest-energy motif of free clusters, were selected for studying the selective colorimetric detection of sulphide species, including S, SH, Cys, and H2S. It should be noted that the icosahedral morphology has been proved to be more stable compared with other morphologies (cuboctahedron and decahedron) for small Ag–Au nanoalloys at the DFT level.31–33 The lowest-energy atomic ordering of 55-atom Ag–Au clusters used in this work was obtained from Monte Carlo simulations at the empirical potential level, and then subjected to DFT relaxation. The details of Monte Carlo simulations can be found in our previous work.34–36 The interaction between metal atoms was modeled empirically based on the well-established EAM potential.37–40 The resulting configurations from Monte Carlo simulations were used to study the adsorption properties.
As shown in Fig. 1a, the Ag42Au13 cluster with the icosahedral structure contains a 13-atom Au core and 42-atom Ag shell, termed as the magical structure, agreeing with the core–shell structure in experiments.41–44 It is also noted that the Ag42Au13 cluster with a gold atom on the surface is more stable than the Ag42Au13 cluster with a gold atom in the core. In addition, the morphologies for S, SH and H2S were optimized at the DFT level, which have been used elsewhere.45 Compared with the R–COOH and R–NH2 groups in the Cys, the SH one can bind to both the Ag/Au surfaces46,47 and Au55 cluster48 more tightly. Thus, only the binding of the SH group in the Cys with the Ag42Au13 and Ag55 clusters was studied in our calculations. As is well-known, the icosahedral structure with highly symmetry can be divided into twenty equivalent triangular faces. As shown in Fig. 1a, six different adsorption sites are found for each triangular face: B1 (between one edge and one vertex atom), B2 (between the two edge atoms), H1 (on the hollow site among two edge and one vertex atom), H2 (on the hollow site among three edge atoms), T1 (on the top of vertex), T2 (on the top of the edge atom).
The adsorption energy of S (Eads) on these Ag–Au clusters at different adsorption sites is defined by
 | (1) |
3. Results and discussions
3.1 Sensitivity
Table 1 lists the initial and final adsorption sites, and adsorption energies (Eads, in eV) at all possible adsorption sites for the adsorption of S, SH, Cys and H2S on the Ag42Au13 and Ag55 clusters. In addition, the adsorption energies at the most favorite adsorption sites are given in italic and bold letters (see Table 1) and the corresponding configurations are shown in Fig. 1b. For all the cases, the adsorption strength of those sulphide species on the Ag42Au13 cluster is stronger than those on the Ag55 cluster, indicating that the Ag42Au13 cluster shows more intense sensitivity for those sulphide species than the Ag55 one. This may be due to the synergistic effect between Ag and Au that introduces redistribution of electronics between Ag and Au atoms. Compared with the Ag55 cluster, shorter atom-to-atom average distance between the sulphide species and adsorbed Ag atoms can be observed for the Ag42Au13 cluster (see Table 1). In addition, the average bond lengths were calculated regarding the Au–Au/Ag bonds of core Au13 atoms in the Ag42Au13 cluster and the Ag–Ag bonds of core Ag13 atoms in the Ag55 cluster, as listed in Table 2. It can be found that the average bond length of the Au–Au/Ag bonds in the Ag42Au13 cluster is larger than that of the core Ag13 atoms in the Ag55 cluster. In addition, the average bond length changes about 0.022 and 0.048 Å for the Ag42Au13 and Ag55 clusters upon the adsorption of S, respectively. It indicates that the adsorption of S leads to less changes in the tension of Au–Au/Ag bonds compared to the Ag–Ag ones.| Sensor | Adsorbate | Adsorption site | Eads (eV) | D (Å) | |
|---|---|---|---|---|---|
| Initial | Final | ||||
| Ag55 | S | B1 | H1 | −4.302 | 2.449 |
| B2 | H2 | −4.208 | 2.473 | ||
| H1 | H1 | −4.304 | 2.455 | ||
| H2 | H2 | −4.211 | 2.474 | ||
| T1 | T1 | −3.694 | 2.434 | ||
| T2 | H1 | −4.208 | 2.449 | ||
| SH | B1 | B1 | −2.682 | 2.516 | |
| B2 | B2 | −2.563 | 2.533 | ||
| H1 | B2 | −2.667 | 2.533 | ||
| H2 | H2 | −2.438 | 2.587 | ||
| T1 | T1 | −2.794 | 2.070 | ||
| T2 | B2 | −2.683 | 2.516 | ||
| Cys | B1 | B2 | −2.301 | 2.533 | |
| B2 | B2 | −2.636 | 2.575 | ||
| H1 | H1 | −2.259 | 2.621 | ||
| H2 | H1 | −2.429 | 2.622 | ||
| T1 | T1 | −1.726 | 2.426 | ||
| T2 | T2 | −1.787 | 2.410 | ||
| H2S | B1 | T2 | −0.306 | 2.740 | |
| B2 | T2 | −0.292 | 2.713 | ||
| H1 | T2 | −0.301 | 2.723 | ||
| H2 | T2 | −0.256 | 2.750 | ||
| T1 | T1 | −0.366 | 2.521 | ||
| T2 | T2 | −0.301 | 2.692 | ||
| Ag42Au13 | S | B1 | H1 | −4.418 | 2.465 |
| B2 | H1 | −4.416 | 2.466 | ||
| H1 | H1 | −4.416 | 2.465 | ||
| H2 | H2 | −4.269 | 2.482 | ||
| T1 | T1 | −4.095 | 2.383 | ||
| T2 | H1 | −4.420 | 2.463 | ||
| SH | B1 | B1 | −2.775 | 2.543 | |
| B2 | B2 | −2.874 | 2.549 | ||
| H1 | B2 | −2.666 | 2.548 | ||
| H2 | B2 | −2.671 | 2.545 | ||
| T1 | T1 | −3.209 | 2.476 | ||
| T2 | B1 | −2.870 | 2.534 | ||
| Cys | B1 | B1 | −2.909 | 2.527 | |
| B2 | B2 | −2.411 | 2.553 | ||
| H1 | H1 | −2.495 | 2.555 | ||
| H2 | H2 | −2.374 | 2.634 | ||
| T1 | T1 | −2.009 | 2.404 | ||
| T2 | H1 | −3.006 | 2.653 | ||
| H2S | B1 | T2 | −0.351 | 2.664 | |
| B2 | T2 | −0.352 | 2.676 | ||
| H1 | T2 | −0.361 | 2.662 | ||
| H2 | T2 | −0.354 | 2.651 | ||
| T1 | T1 | −0.473 | 2.515 | ||
| T2 | T2 | −0.326 | 2.647 | ||
| Average bond length (Å) Au–Au/Ag | Average bond length (Å) Ag–Ag | ||
|---|---|---|---|
| Ag42Au13 | 2.949 | Ag55 | 2.910 |
| Ag42Au13–S | 2.927 | Ag55–S | 2.862 |
| Ag42Au13–SH | 2.928 | Ag55–SH | 2.864 |
| Ag42Au13–Cys | 2.930 | Ag55–Cys | 2.866 |
| Ag42Au13–H2S | 2.934 | Ag55–H2S | 2.869 |
To understand the synergistic effect between Ag and Au on the adsorption of sulphide species, taking the Ag42Au13 cluster as an example, Fig. 2 shows the partial densities of states (PDOSs) of d orbitals of core Au13 atoms of the Ag42Au13 cluster and corresponding core Au13 atoms in the vacuum. As shown in Fig. 2, upon the adsorption of sulphide species, the electronic states of the core Au13 atoms shift toward the Fermi level in the PDOSs. Accordingly, the average bond lengths of Au–Au bonds in the Ag42Au13 cluster change from 2.947 to 2.949 Å upon the adsorption of S. It means that the shift of the electronic states of the core Au13 atoms toward the Fermi level is associated with the stretched Au–Au bonds, which has been proved in previous work.49–51 It can be observed that new peaks occur in the range from −7 to −5 eV in the PDOSs of d orbitals of core Au13 atoms in the Ag42Au13 cluster, indicating that the synergistic effect introduces new electronic states of core Au13 atoms. Compared with the vacuum case, the peaks split into a set of decreased peaks from −5 to −2 eV in the PDOSs of d orbitals of core Au13 atoms in the Ag42Au13 cluster, suggesting new bond formations occur between Au atoms and adjacent Ag atoms of the shell of the Ag42Au13 cluster. These results show that the synergistic effect plays an important role in the electronic states of the Ag42Au13 cluster, which leads to the stronger adsorption compared to the Ag55 cluster.
In order to get a deep insight to the synergistic effect between Ag and Au on the adsorption strength of sulphide species, further analysis about the charge distribution differences of the Ag42Au13 and Ag55 clusters are calculated and plotted in Fig. 3. Although various sulphide species lead to various charge transfer of the core atoms for the Ag42Au13 and Ag55 clusters, more charge transfer of the core atoms can be observed for the Ag42Au13 cluster compared to the Ag55 case. This is the result of the synergistic effect between Ag and Au, which leads to more stable electronic states of the Ag42Au13 cluster than the Ag55 cluster. Taking the adsorption of S as an example, considerable charge transfer of core Au atoms corresponds to the great changes in the PDOSs of d orbitals of core Au13 atoms of the Ag42Au13 cluster. However, for the Ag55 cluster, less charge transfer is found for core Ag atoms of the Ag55 cluster, suggesting that the synergistic effect between Ag and Au leads to more hybridization between Ag and Au in the electronic states. It should be noted that, for the adsorption of Cys, the great charge transfer of core Au13 atoms, especially for the asymmetric charge transfer of the Au atom close to Cys, leads to the intense and extraordinary peak at −2.3 eV in the PDOSs of d orbitals of core Au13 atoms of the Ag42Au13 cluster. In addition to the charge distribution results, the Löwdin charge analysis shown in Table 3 provides the verification of the hybridization between Ag and Au in the electronic states. It can be observed that the average charge transfer of core Au atoms of the Ag42Au13 cluster is around 0.96 e, and the average charge transfer of core Ag atoms of the Ag55 cluster is around 0.19 e. It should be noted that core Au13 atoms in the Ag42Au13 cluster accept negative charge in average, and core Ag13 atoms of the Ag55 cluster donate negative charge in average. Our results verify that more hybridization between Ag and Au leads to stronger adsorption strength of the Ag42Au13 cluster compared to the Ag55 cluster.
| Average charge transfer (e) of core Au atoms | Average charge transfer (e) of core Ag atoms | ||
|---|---|---|---|
| Ag42Au13–S | 0.96 | Ag55–S | −0.19 |
| Ag42Au13–SH | 0.98 | Ag55–SH | −0.19 |
| Ag42Au13–Cys | 0.96 | Ag55–Cys | −0.19 |
| Ag42Au13–H2S | 0.97 | Ag55–H2S | −0.19 |
3.2 Selectivity
Since the selectivity of adsorption-based colorimetric sensor can be determined by the adsorption strength, the adsorption energies of the Ag42Au13 and Ag55 clusters towards S, SH, Cys, and H2S at all possible adsorption sites are calculated and listed in Table 1. Fig. 4 plots the adsorption energies of the Ag42Au13 and Ag55 clusters towards S, SH, Cys, and H2S at the most favorite adsorption site and the corresponding configurations are shown in Fig. 1b. It is found that the adsorption strength of the Ag42Au13 and Ag55 clusters towards sulphide species at the most favorite adsorption site follows the same order of S > SH > Cys > H2S, indicating that both the Ag42Au13 and Ag55 clusters possess high selectivity for the colorimetric detection of S over other sulphide species. In addition, the adsorption strength of the Ag42Au13 and Ag55 clusters towards SH is close to the Cys case, indicating that the valence state of S may play a role in the affinity between S and adsorbed Ag atoms. Moreover, the adsorption strength of H2S on the Ag42Au13 cluster is close to 0 eV, indicating little adsorption affinity between adsorbed Ag and S atoms, which may be the result of zero valence of the S atom. As can be observed from Table 2, the adsorption of sulphide species could lead to the decrease of the average bond lengths of both the Au–Au/Ag bonds of core Au13 atoms in the Ag42Au13 cluster and the Ag–Ag bonds of core Ag13 atoms in the Ag55 cluster. In addition, the corresponding average bond lengths increase in the order of S < SH < Cys < H2S, which agrees well with the decrease of the adsorption energy in the order of S > SH > Cys > H2S. It can be concluded that the adsorption leads to the changes in the tension of bonds of the core atoms in the clusters, and the tension decreases with the decrease of the adsorption strength. This is also a result of re-distribution of electronic properties of the Ag55 and Ag42Au13 clusters, which could be further explained by the results of PDOSs as follows. | ||
| Fig. 4 Adsorption energies (Eads, in eV) of the Ag55 and Ag42Au13 clusters towards S, SH, Cys and H2S at the most favorable adsorption site. | ||
To understand the trend in the adsorption strength of the Ag42Au13 and Ag55 clusters toward sulphide species, taking the Ag42Au13 cluster as an example, the PDOSs of p orbitals of S atom of sulphide species and corresponding S atom of sulphide species in vacuum are plotted in Fig. 5. It can be observed from the PDOSs of p orbitals of S atom of sulphide species in vacuum possess two typical peaks at −1.7 and 0 eV. Obviously, the peak at 0 eV maintains for the adsorption of various sulphide species. However, the peak at −1.7 eV splits into less intense peaks with the adsorption of various sulphide species. For example, the peak splits into a distinct peak above −1.7 eV and a set of weak peaks, indicating that the hybridization between S and adsorbed Ag atoms leads to new electronic state that close to the Fermi energy state. In addition, the distinct peak shifts to −1.9, −2.0, and −4.9 eV for SH, Cys, and H2S, respectively, suggesting that the peak changes in the same order of the adsorption. This point indicates that strong adsorption strength of sulphide species can be elucidated by the close location to the Fermi energy of the distinct peak in the PDOSs of p orbitals of S atoms. Accordingly, notable electronic contribution of S at high energy to the integral electronic states of the cluster determines the adsorption strength of various sulphide species. The Löwdin charge analysis in Table 4 verifies the results explicitly. The charge transfer of S atom for various sulphide species follows the order of S > SH > Cys > H2S, which agrees well with the order of the adsorption strength of S > SH > Cys > H2S. Therefore, it can be concluded that the atomic orbital of S occupies higher energy of the molecular orbital, leading to more charge transfer of S atoms, which further influence the adsorption strength of sulphide species.
| Average charge transfer (e) of S atom | |
|---|---|
| Ag42Au13–S | 0.75 |
| Ag42Au13–SH | 0.55 |
| Ag42Au13–Cys | 0.38 |
| Ag42Au13–H2S | 0.06 |
In order to understand the coverage effect of adsorbed S on the Ag–Au nanoalloys for detecting sulphide species as colorimetric sensors, the adsorptions of S with the number of 2, 4, 6, 8, 10 and 12 at the T1 adsorption site of the Ag42Au13 cluster with a good symmetry are calculated, as shown in Fig. 6. Fig. 7 plots the adsorption energies with changing the number of adsorbed S on the Ag42Au13 cluster. It can be found that the adsorption energies decrease with increasing the coverage of S. In addition, the adsorption strength of the Ag42Au13 cluster toward 12S is the weakest one compared to other cases, indicating that the coverage affects the sensitivity of the Ag42Au13 cluster.
 | ||
| Fig. 7 Adsorption energies (Eads, in eV) of the Ag42Au13 cluster with changing the number of adsorbed S at the T1 adsorption site after the local relaxation at the DFT level. | ||
In order to understand the coverage effect deeply, the charge difference distribution differences of the Ag42Au13 cluster with the adsorption of 2S and 4S are calculated and plotted in Fig. 8. As shown in Fig. 8a, the positive charge distributes midway between S and adsorbed Ag atom for the adsorption of 2S. However, more positive charge can be found between S and adsorbed Ag atoms for the 4S case, meaning that more positive charge transfers from Ag to S compared to the 2S case. It can be concluded that increasing the coverage of S could introduce more charge transfer between S and adsorbed Ag atoms. It should be noted that the irregular charge distribution could be observed from Fig. 8b, which is the result of the adsorption of S at adjacent sites. This indicates that increasing the coverage of S could introduce more complicated charge transfer between S and adsorbed Ag atoms. Therefore, increasing the coverage of S on the Ag42Au13 cluster leads to the decrease of the sensitivity toward S, which should be taken into consideration when preparing the Ag–Au nanoalloys for detecting sulphide species.
4. Conclusions
In summary, the sensing mechanism and selectivity for the colorimetric detection of sulphide (S) among sulphide species, such as S, SH, cysteine (Cys), and H2S, using Ag–Au nanoalloys are investigated by density functional theory (DFT) calculations. It is found that the adsorption strength of S, SH, Cys, and H2S on the Ag42Au13 cluster is stronger than that on the Ag55 one, corresponding to the higher sensitivity of the Ag42Au13 cluster towards sulphide species compared with the Ag55 one. By probing the partial densities of states (PDOSs) and charge difference distribution, the introduction of sulphide species to the Ag42Au13 cluster induces great changes in the electronic properties due to the synergistic effect between Ag and Au, resulting in stronger adsorption strength compared to the Ag55 cluster. In addition, the adsorption strength of the Ag42Au13 and Ag55 clusters towards sulphide species follows the order of S > SH > Cys > H2S, indicating that both the Ag42Au13 and Ag55 clusters process high selectivity for the colorimetric detection of S over other sulphide species. By investigating the PDOSs and charge difference distribution, the high selectivity of the Ag42Au13 cluster towards S over other sulphide species is determined by the electronic contribution of S atom of various sulphide species to the integral electronic states of the cluster. Moreover, the coverage effect of S on the Ag42Au13 cluster is investigated by analyzing the charge distribution between S and adsorbed Ag atoms. It is verified that increasing the coverage of S leads to the decrease of the adsorption strength. Our theoretical results are expected to provide insights into the sensing mechanism of Ag–Au nanoalloys and guidelines to design even more powerful sensors for detecting S in live cells.Acknowledgements
This work is supported by the National Natural Science Foundation of China (21576008, 91334203), Beijing Higher Education Young Elite Teacher Project, BUCT Fund for Disciplines Construction and Development (Project No. XK1501), Fundamental Research Funds for the Central Universities (Project No. buctrc201530), “Chemical Grid Project” of BUCT and Supercomputing Center of Chinese Academy of Sciences (SCCAS).References
- S. Chen, Z.-j. Chen, W. Ren and H.-w. Ai, J. Am. Chem. Soc., 2012, 134, 9589–9592 CrossRef CAS PubMed
.
- H. Y. Jung, Y. L. Kim, S. Park, A. Datar, H.-J. Lee, J. Huang, S. Somu, A. Busnaina, Y. J. Jung and Y.-K. Kwon, Analyst, 2013, 138, 7206–7211 RSC
.
- K. Sasakura, K. Hanaoka, N. Shibuya, Y. Mikami, Y. Kimura, T. Komatsu, T. Ueno, T. Terai, H. Kimura and T. Nagano, J. Am. Chem. Soc., 2011, 133, 18003–18005 CrossRef CAS PubMed
.
- L. Gell, L. Lehtovaara and H. Häkkinen, J. Phys. Chem. A, 2014, 118, 8351–8355 CrossRef CAS PubMed
.
- Y. Qian, J. Karpus, O. Kabil, S.-Y. Zhang, H.-L. Zhu, R. Banerjee, J. Zhao and C. He, Nat. Commun., 2011, 2, 495 CrossRef PubMed
.
- A. R. Lippert, E. J. New and C. J. Chang, J. Am. Chem. Soc., 2011, 133, 10078–10080 CrossRef CAS PubMed
.
- C. Yu, X. Li, F. Zeng, F. Zheng and S. Wu, Chem. Commun., 2013, 49, 403–405 RSC
.
- B. Xiong, L. Peng, X. Cao, Y. He and E. S. Yeung, Analyst, 2015, 140, 1763–1771 RSC
.
- L. A. Montoya, T. F. Pearce, R. J. Hansen, L. N. Zakharov and M. D. Pluth, J. Org. Chem., 2013, 78, 6550–6557 CrossRef CAS PubMed
.
- J. Hao, B. Xiong, X. Cheng, Y. He and E. S. Yeung, Anal. Chem., 2014, 86, 4663–4667 CrossRef CAS PubMed
.
- H.-H. Deng, S.-H. Weng, S.-L. Huang, L.-N. Zhang, A.-L. Liu, X.-H. Lin and W. Chen, Anal. Chim. Acta, 2014, 852, 218–222 CrossRef CAS PubMed
.
- J. M. Obliosca, C. Liu and H.-C. Yeh, Nanoscale, 2013, 5, 8443–8461 RSC
.
- A. Hung, S. Mwenifumbo, M. Mager, J. J. Kuna, F. Stellacci, I. Yarovsky and M. M. Stevens, J. Am. Chem. Soc., 2011, 133, 1438–1450 CrossRef CAS PubMed
.
- L. Zhang, Y. Li, D.-W. Li, C. Jing, X. Chen, M. Lv, Q. Huang, Y.-T. Long and I. Willner, Angew. Chem., Int. Ed., 2011, 50, 6789–6792 CrossRef CAS PubMed
.
- K. Li, W. Wang and D. Cao, J. Phys. Chem. C, 2011, 115, 12015–12022 CAS
.
- C. Fasciani, M. J. Silvero, M. A. Anghel, G. A. Argüello, M. C. Becerra and J. C. Scaiano, J. Am. Chem. Soc., 2014, 136, 17394–17397 CrossRef CAS PubMed
.
- Z. Chen, J. Li, X. Chen, J. Cao, J. Zhang, Q. Min and J.-J. Zhu, J. Am. Chem. Soc., 2015, 137, 1903–1908 CrossRef CAS PubMed
.
- Y. Zhao, L. Liu, H. Kuang, L. Wang and C. Xu, RSC Adv., 2014, 4, 56052–56056 RSC
.
- R. Jin and K. Nobusada, Nano Res., 2014, 7, 285–300 CrossRef CAS
.
- W. Zhang, J. Zheng, C. Tan, X. Lin, S. Hu, J. Chen, X. You and S. Li, J. Mater. Chem. B, 2015, 3, 217–224 RSC
.
- A. H. Pakiari and Z. Jamshidi, J. Phys. Chem. A, 2010, 114, 9212–9221 CrossRef CAS PubMed
.
- L. G. AbdulHalim, N. Kothalawala, L. Sinatra, A. Dass and O. M. Bakr, J. Am. Chem. Soc., 2014, 136, 15865–15868 CrossRef CAS PubMed
.
- Z. Zhang, Z. Chen, S. Wang, C. Qu and L. Chen, ACS Appl. Mater. Interfaces, 2014, 6, 6300–6307 CAS
.
- P. D. Howes, R. Chandrawati and M. M. Stevens, Science, 2014, 346, 1247390 CrossRef PubMed
.
- B. Xiong, R. Zhou, J. Hao, Y. Jia, Y. He and E. S. Yeung, Nat. Commun., 2013, 4, 1708 CrossRef PubMed
.
- S. A. Haque, R. L. Bolhofner, B. M. Wong and M. A. Hossain, RSC Adv., 2015, 5, 38733–38741 RSC
.
- X.-Y. Pang, C. Wang, Y.-H. Zhou, J.-M. Zhao and G.-C. Wang, J. Mol. Struct.: THEOCHEM, 2010, 948, 1–10 CrossRef CAS
.
- G. Paolo, B. Stefano, B. Nicola, C. Matteo, C. Roberto, C. Carlo, C. Davide, L. C. Guido, C. Matteo, D. Ismaila, C. Andrea Dal, G. Stefano de, F. Stefano, F. Guido, G. Ralph, G. Uwe, G. Christos, K. Anton, L. Michele, M.-S. Layla, M. Nicola, M. Francesco, M. Riccardo, P. Stefano, P. Alfredo, P. Lorenzo, S. Carlo, S. Sandro, S. Gabriele, P. S. Ari, S. Alexander, U. Paolo and M. W. Renata, J. Phys.: Condens. Matter, 2009, 21, 395502 CrossRef PubMed
.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed
.
- M. Methfessel and A. T. Paxton, Phys. Rev. B: Condens. Matter Mater. Phys., 1989, 40, 3616–3621 CrossRef CAS
.
- H.-J. Li and J.-J. Ho, J. Phys. Chem. C, 2012, 116, 13196–13201 CAS
.
- J. L. F. Da Silva, H. G. Kim, M. J. Piotrowski, M. J. Prieto and G. Tremiliosi-Filho, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 205424 CrossRef
.
- C. M. Chang, C. Cheng and C. M. Wei, J. Chem. Phys., 2008, 128, 124710 CrossRef CAS PubMed
.
- D. Cheng, S. Huang and W. Wang, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 064117 CrossRef
.
- D. Cheng, X. Liu, D. Cao, W. Wang and S. Huang, Nanotechnology, 2007, 18, 475702 CrossRef
.
- D. Cheng and W. Wang, Nanoscale, 2012, 4, 2408–2415 RSC
.
- S. M. Foiles, M. I. Baskes and M. S. Daw, Phys. Rev. B: Condens. Matter Mater. Phys., 1986, 33, 7983–7991 CrossRef CAS
.
- D. Cheng, I. S. Atanasov and M. Hou, Eur. Phys. J. D, 2011, 64, 37–44 CrossRef CAS
.
- R. A. Johnson, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 41, 9717–9720 CrossRef CAS
.
- R. A. Johnson, Phys.
Rev. B: Condens. Matter Mater. Phys., 1989, 39, 12554–12559 CrossRef
.
- K. K. Haldar, S. Kundu and A. Patra, ACS Appl. Mater. Interfaces, 2014, 6, 21946–21953 CAS
.
- A. K. Samal, L. Polavarapu, S. Rodal-Cedeira, L. M. Liz-Marzán, J. Pérez-Juste and I. Pastoriza-Santos, Langmuir, 2013, 29, 15076–15082 CrossRef CAS PubMed
.
- S. Anandan, F. Grieser and M. Ashokkumar, J. Phys. Chem. C, 2008, 112, 15102–15105 CAS
.
- S. Guha, S. Roy and A. Banerjee, Langmuir, 2011, 27, 13198–13205 CrossRef CAS PubMed
.
- L. Ling, R. Zhang, P. Han and B. Wang, Fuel Process. Technol., 2013, 106, 222–230 CrossRef CAS
.
- N. B. Luque, P. Velez, K. Potting and E. Santos, Langmuir, 2012, 28, 8084–8099 CrossRef CAS PubMed
.
- S. Fischer, A. C. Papageorgiou, M. Marschall, J. Reichert, K. Diller, F. Klappenberger, F. Allegretti, A. Nefedov, C. Wöll and J. V. Barth, J. Phys. Chem. C, 2012, 116, 20356–20362 CAS
.
- J. A. Carr, H. Wang, A. Abraham, T. Gullion and J. P. Lewis, J. Phys. Chem. C, 2012, 116, 25816–25823 CAS
.
- E. Z. da Silva, F. D. Novaes, A. J. R. da Silva and A. Fazzio, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 115411 CrossRef
.
- E. P. M. Amorim and E. Z. d. Silva, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 115463 CrossRef
.
- A. J. Simbeck, N. Lanzillo, N. Kharche, M. J. Verstraete and S. K. Nayak, ACS Nano, 2012, 6, 10449–10455 CAS
.
| This journal is © The Royal Society of Chemistry 2016 |