Utilization of renewable bio-based resources, viz. sorbitol, diol, and diacid, in the preparation of two pack PU anticorrosive coatings†
Abhijeet Ananda,
Ravindra D. Kulkarnib,
Chandrashekhar K. Patila and
Vikas V. Gite*a
aSchool of Chemical Sciences, North Maharashtra University, Jalgaon-425 001, M. S., India. E-mail: vikasgite123@gmail.com
bUniversity Institute of Chemical Technology, North Maharashtra University, Jalgaon-425 001, M. S., India
First published on 8th January 2016
Abstract
The present research work reveals the use of renewable ingredients to synthesize the polyester polyol part of polyurethanes as a modification different from the traditional pattern via the melt condensation technique. The difunctional acid used in the preparation of polyester polyol was selected according to the chain length and molecular weight. Both renewable and non-renewable difunctional acids were used for comparing the coating properties of each formulation. Chemical and corrosion resistances were also studied with respect to their effects on final coating properties in terms of their structural parameters for sustainable production of 2K PU coatings. Synthesized coatings were further characterized by techniques like gloss, adhesion, flexibility, and pencil hardness. Overall, sebacic acid based formulation was found to be most suitable among all for coating performance, especially anticorrosive property, gloss, and pencil hardness.
Introduction
Polyurethane (PU) is a versatile class of tailor made polymers used in the paint and coating sectors for a very long time due to high resistivity against chemicals along with excellent mechanical properties.1,2 They are also used in day-to-day life for manufacturing of shock absorbers, elastomers, sealants, foams, automobiles, roofs, biomedical materials, appliances in thermoplastic PUs, etc.3–6 To date, most applications of PU are based on conventional materials, which have severe drawbacks towards the environment and human health along with the economic point of view. To overcome aforesaid problems, nowadays, an increasing demand for renewable source based technology has been observed in the preparation of paints and coatings.7–9 Some reasons for this approach are concerned with preventing greenhouse gas emission, nontoxic by-product, good biocompatibility, higher sustainability of raw materials, etc.10–13 Two ways to apply the approach are, first, the raw material used for formulations should be renewable or derived from renewable material and the second is to minimize the use of organic solvents or to prefer green solvents.14,15 A new trend currently is to use sugar alcohol and vegetable oil based ingredients in the coating sector. Hence a lot of experiments are being carried out to apply these considerations via eco-friendly approaches16–19 because, as per commercial requirements in the industrial sector, PU possesses robust physical properties compared to other formulations. Among all available renewable sources, maximum efforts are taken to use vegetable oil for preparation of bio-based PU20–23 and very much less work is carried out on sorbitol due to phase separation and brittle nature of polymeric resins based on it.24 Therefore, it was decided to conduct synthetic work related to sugar alcohol based building block preparation in the form of protective coating and find a remedy for related problems. The purpose for the said coating is to develop a barrier for restricting the contact between metals with exterior exposures to prevent deterioration in the form of corrosion by atmospheric contaminants. It is also non-economical for commercial sectors to bear huge regular losses caused by corrosion of substrates and maintenance of the same.Developing research and increasing technological trend diminished the alternative utilization of one hexafunctional sugar alcohol, i.e. sorbitol, in the coating sector rather than its limited use as an artificial sweetener. The technology for enzymatic and chemical conversion of polysaccharides to sorbitol is very well established and reported in the literature.25,26 One of the promising features of carbohydrates is that they can form a cross-linking network like an interpenetrating polymer network in PU coatings due to strong interfacial bonding.27 Another reason for considering sorbitol for preparation of renewable based polyols is its superior physical properties, i.e. thermal stability, solvent resistance, and mechanical properties, due to its polyhydroxy structure.28
To generalize the above discussion, the research and modifications presented in resin formulations are based on renewable raw materials, viz. sorbitol, diacids, and 1,4-butanediol. Sorbitol comprises the main part in formulations to prepare polyester polyol compared to the others. The process was optimized via melt condensation and further developed for the preparation of PU coatings with consideration that the molecular structure of PU depends upon the compound used, polymerization techniques, stoichiometric ratio of selected compounds, etc.
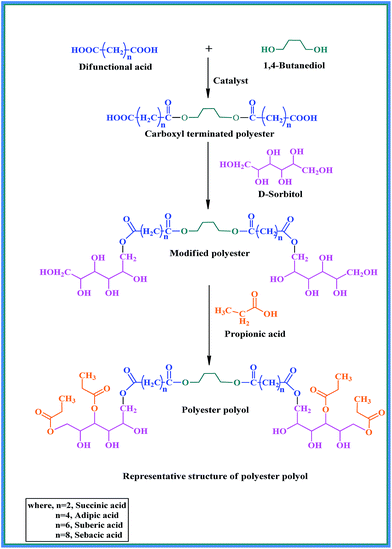
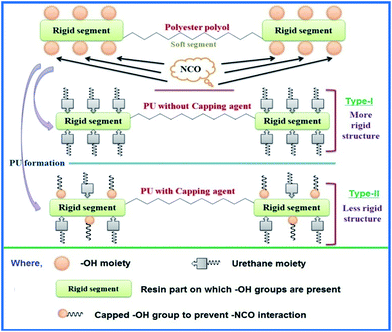
A series of polyester polyols was prepared comprising five components, i.e. one difunctional acid (succinic acid, adipic acid, suberic acid, sebacic acid), one monofunctional acid (propionic acid), two multifunctional hydroxyl compounds (sorbitol and 1,4-butanediol) with p-toluenesulphonic acid (PTSA) as a catalyst. Different aliphatic dicarboxylic acids and 1,4-butanediols were used to introduce flexibility to the polyols, and all of them can be of renewable origin except adipic acid as a non-renewable ingredient. The whole reaction was performed in three steps. The purpose of the different steps was to add a new ingredient successively at different reaction temperature for a specific purpose. In the first two steps, our aim was to increase the chain length along with molecular weight of the prepolymer. In the final step, a few of the excess hydroxyl groups of sorbitol were blocked by propionic acid as capping agent to avoid more consumption of diisocyanate for economic and health reasons as well as to control hard segment and cross-linking sites.
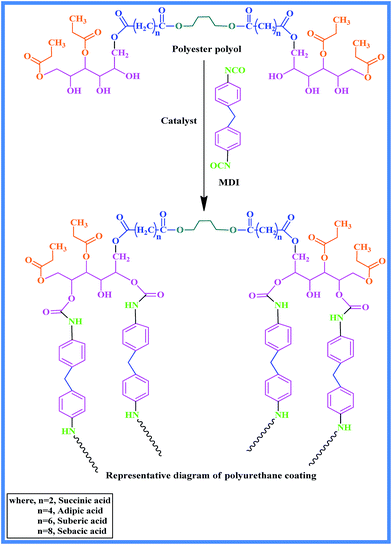
The prepared polyester polyols were further reacted with 4,4′-methylenebis(phenylisocyanate) (MDI) for preparation of two pack PU coatings. The prepared PU coatings were characterized for their gloss, flexibility, adhesion, pencil hardness, mar resistance, etc. The final material comprises hard and soft segments29,30 and one needs to study the effect of the change in difunctional acid on soft and hard segments.
Experimental
Materials
Dibutyltindilaurate (DBTDL) and PTSA were received from Himedia and Molychem, respectively. Sorbitol, succinic acid, suberic acid, sebacic acid, and 1,4-butanediol were supplied by S D Fine Chem. Ltd, Mumbai, India. MDI, adipic acid, and propionic acid were provided by Merck. All chemicals were used as received and without any further purification.Preparation of polyester polyol
The esterification of renewable ingredients was carried out by following the experimental procedure as reported in our previous attempts.24 A three neck round bottom flask was equipped in the assembly set up for preparation of polyester polyol with a nitrogen inlet, thermometer, distilling bridge, and overhead stirrer. Initially it was charged with one of the difunctional acids (as per the formulation), i.e. succinic acid, adipic acid, suberic acid, sebacic acid (1 M), and 1,4-butanediol (0.5 M) by maintaining 120–140 °C temperature in the presence of PTSA (0.1% of total mass of ingredients) as a catalyst to accelerate the esterification reaction. Sorbitol (2 M) was added within 60–90 min after increasing the temperature to 160 °C, when the first intermediate product attained an acid value of half the initial. After this, stoichiometric amount of propionic acid (4 M) was added to the reaction mixture as a third step addition for the desired product. The final temperature was adjusted between 180 and 190 °C and from time to time acid value was monitored throughout the experiment (Scheme 1). Reaction was stopped when the acid value fell near to 10, indicating consumption of maximum numbers of carboxylic moieties. Other details regarding the yield/conversion, acid value and hydroxyl value for each resultant product and different successive steps are mentioned in Table S1 (ESI†).Preparation of PU coatings
By mixing of polyester polyol and MDI in accordance with a stoichiometric ratio of 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for NCO/OH, ingredients were used for preparation of two pack (2K) PU coatings.31 A series of coatings were prepared using different polyester polyols synthesized from renewable and non-renewable acids (Scheme 2). Use of cyclohexanone as a moderate solvent at 75 wt% in terms of polyol content was utilized for complete dissolution of polyester polyol in the presence of DBTDL catalyst for prevention of the problems related to phase separation and proper mixing to reduce viscosity of the medium. Then the coatings were applied on mild steel panels having a size of 3 × 6 inch by means of a brush or bar applicator. For 48 h, the panels were cured at room temperature under observation and were kept at normal conditions.
1 for NCO/OH, ingredients were used for preparation of two pack (2K) PU coatings.31 A series of coatings were prepared using different polyester polyols synthesized from renewable and non-renewable acids (Scheme 2). Use of cyclohexanone as a moderate solvent at 75 wt% in terms of polyol content was utilized for complete dissolution of polyester polyol in the presence of DBTDL catalyst for prevention of the problems related to phase separation and proper mixing to reduce viscosity of the medium. Then the coatings were applied on mild steel panels having a size of 3 × 6 inch by means of a brush or bar applicator. For 48 h, the panels were cured at room temperature under observation and were kept at normal conditions.
Characterization
1H-NMR spectra were scanned at 300 MHz with a Varian Mercury YH-300 FT-NMR in CDCl3 using TMS as an internal standard. The chemical shift values are on δ scale. FT-IR spectra were scanned by means of a Shimadzu-8400 FT-IR spectrometer in the range of 4000 to 450 cm−1 by the NaCl thin plate method using nujol as a solvent. Molecular weight of samples was measured by an Agilent GPC 1260 Infinity, USA, with an Agilent PL gel 5 μm mixed-C column (300 × 7.5 mm, 5 μm). Tetrahydrofuran was used as an eluent at 1 mL per min flow rate at 30 °C using polystyrene as a standard reference. A thermogravimetric analyser (TGA) and differential scanning calorimetric (DSC) analyser (Perkin-Elmer-4000 series) were used for studying thermal stability and behaviour of PU coatings, respectively. The samples were analysed using N2 at a flow rate of 20 mL min−1 for inert atmosphere with a heating rate of 10 °C min−1. Temperature range for TGA was 30–800 °C, while for DSC it was −20 to 170 °C.An electrochemical analyzer PGSTAT 30 differential electrometer manufactured by Metrohm, India was used to execute the electrochemical study and for determination of the corrosion rate. An electrochemical cell with three electrodes, viz. a saturated calomel electrode as a reference electrode, platinum wire as a counter electrode, and coated panel as a working electrode, was used for electrochemical study with 3.5% NaCl solution as electrolyte. Prior to examination, panels were polished with sand papers, followed by thorough rinsing with solvent and double distilled water with exposure in air to dry. Epoxy adhesive (Araldite) was applied over the PU sample panels with attachment of conductive wire from the other side to restrict the defects arising due to salt solution and to provide current, respectively. Coated panels having an area of 1 cm × 1 cm were immersed in solution for at least 20 min before analysis.
Coating properties
The cross-cut adhesion test was carried out for adhesion testing of PU coatings applied on mild steel panels as per ASTM standard D-3359-95B. An Elcometer 107 X-hatch type apparatus was used to perform the test. A 1 mm × 1 mm size of cross cut mark was applied over the sample using 10 point sharp razor blades of the cross hatch tester. Then an adhesive tape was adhered over the sectional surface. After a few minutes, the tape was stripped off rapidly. The number of cubes removed by the adhesive tape was compared with the remaining cubes on the surface. An automatic digital display gloss meter was used for testing the gloss of coated samples after calibrating with a standard polished black reference panel provided by manufacturer. Then gloss measurement of sample panels was carried out at an angle of 60°.Conical mandrel equipment manufactured by Raj Scientific Instruments, Mumbai was used to perform bending tests. The sample panel was bent at different angles between 45° and 180° to observe any peeling or cracking on the bent portion. A pencil hardness tester was used to evaluate the strength of coating films for their hardness by a pencil number from 1B (very soft) to 6H (very hard). Mar resistance was performed in the laboratory as per ASTM D-6279 standard on a mar resistance tester, and it was denoted by ‘g’ in respect of the term of load and fails to mar the films. Dry film thickness (DFT) of coated panels was measured with an ultrasonic DFT meter, a PosiTector 200 instrument manufactured by Defelsko, USA.
Chemical resistance of PU sample plates was tested for reagents like organic solvent (xylene), acid, and alkali. These films were placed in 5% solutions containing HCl, H2SO4, CH3COOH, NaOH, and NaHCO3. The coating plates were monitored and observed for a time duration of one week for visual appearance at regular intervals of 12 h. Corrosion test was performed by immersing the sample panels in 3.5% NaCl solution and by observing the deterioration of the coating surface. For the study, two types of panels were used, one with an ‘X’ shape cross cut mark and another without a mark. To prevent side corrosion, the side portion of the panels was covered up by tape. The coated panels were tested for a total immersion time of 168 h by continuous inspection after each 24 h.
Results and discussion
A series of polyols was designed to have capping of a few unreacted hydroxyl groups of sorbitol in the final structure of polyester polyols. The reason for using sorbitol was to introduce rigidity and manage the stoichiometric balance in opposition to flexibility generated by aliphatic chain of acid in addition to ester moieties. Sorbitol is also responsible for creating higher cross-linking density in the polymeric network due to its multiple secondary hydroxyl groups. The higher cross-linking density can impart higher thermal resistance to the resultant polymer. The reason for capping of extra hydroxyl groups was to keep minimum hydroxyl groups for further reaction with diisocyanate, which is intended to decrease isocyanate requirement and to reduce hard segments. Propionic acid was used as capping agent to consume some free hydroxyl groups in the second intermediate product to minimize the consumption of excess –NCO as well as to decrease rigidity in final coating properties. In this case, on reaction of propionic acid with unreacted hydroxyl groups of sorbitol, short side branches were introduced which had –CH3 as chain ends. It is clearly seen from the representative structure of a polyester (Fig. 1 and Scheme 1) that it contains diacid and 1,4-butanediol based soft segments, as well as propionic acid based capped side branches containing soft segments due to the presence of ester and –CH2– groups. The presence of additional flexible part and reduction in hard segment (unavailability of –OH groups for formation of urethane as hard segment) are able to balance the soft and hard segments in the resultant polyol. Also, for the purpose of comparison, modified polyester (without capping) was used to convert into PU coating. The coatings required more diisocyanate and were observed to be hazy, brittle, and without uniformity. Hence, capping of a few hydroxyl groups resulted in PU coatings which were free from defects as in earlier cases and as per our hypothetical requirement. Differences between both types of polyols i.e. capped and uncapped are represented in Scheme 1 and their structural changes were compared in 1H-NMR section co-relation with Fig. S2† in the form of capped and uncapped structures. From the comparison, it was found that the capped structure was more prominent and useful rather than uncapped.FT-IR
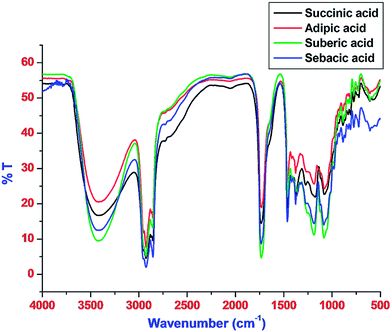
The resultant peaks relating to the reaction between difunctional acid, multifunctional alcohol, and monofunctional acid have been studied by FT-IR analysis and the resulting spectra are shown in Fig. 2. The peak assignments corresponding to each formulation are mentioned in Table S2 (ESI†). The bands near to 3400 cm−1 corresponded to the presence of free –OH group in the structure of polyester polyols. Absorption bands near to 2800 and 2900 cm−1 were due to symmetrical and asymmetrical stretching modes of vibrations corresponding to –CH2– and –CH3 groups.17 A strong band observed near 1730 cm−1 was attributed to![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of ester present in the polyester polyol moiety. The bending vibrations for –CH2– group appeared at frequencies in the range of nearly 1370 cm−1 and 1460 cm−1 for each successive formulation.
O group of ester present in the polyester polyol moiety. The bending vibrations for –CH2– group appeared at frequencies in the range of nearly 1370 cm−1 and 1460 cm−1 for each successive formulation.
1H-NMR
1H-NMR spectra of different polyester polyols show the signal for terminal –CH3 at δ = 1.15 as represented in Scheme 1 for the final-stage product of capped polyester polyol. A different value for –CH2– was observed within a range of δ = 1.2 to 4 due to the neighbouring moiety for which the value ranges from 1.2 to 2.7 corresponding to –CH2– moiety present in between ester groups. H attached to C next to![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O shows its peak towards the downfield region near to 2.5 ppm.32 Due to compact structure and less interparticle distance, the peaks of H atom of the C attached to –OH group were a multiplet. All the NMR spectra are presented in Fig. 3 in sequence related to increasing chain length of difunctional acid based polyester polyols. In the 1H-NMR spectra of polyester polyols prepared in sequence – (a) succinic, (b) adipic, (c) suberic, (d) sebacic acid – each peak corresponded with the prepared polyol structures and positions of protons are denoted by alphabetic characters for better understanding the structure of the capped polyester polyols. As a requirement for more consistent data, 1H-NMR spectrum of each intermediate product was also obtained. 1H-NMR spectra for the first intermediate product, i.e. carboxyl terminated polyester, and second intermediate product, i.e. modified polyester, are shown in Fig. S1 and S2 (ESI†). 1H-NMR spectra from Fig. 3 and S2† were compared to determine the side branches introduced by propionic acid in the final polyester. Peak observed at 1 ppm in the 1H-NMR spectrum of the final polyester was assigned to terminal –CH3 groups. The peak for terminal –CH3 is absent in the 1H-NMR spectrum of the second intermediate product showing that terminal –CH3 groups were absent as shown in Fig. S2.† The overall results indicated that the presence of terminal –CH3 may be due to introduction of side branches as a part of propionic acid. Presence of non-reactive –CH3 groups in replacement of –OH groups can reduce consumption of isocyanate component and reduce the rigid segment of urethane, which matched with our requirements.
O shows its peak towards the downfield region near to 2.5 ppm.32 Due to compact structure and less interparticle distance, the peaks of H atom of the C attached to –OH group were a multiplet. All the NMR spectra are presented in Fig. 3 in sequence related to increasing chain length of difunctional acid based polyester polyols. In the 1H-NMR spectra of polyester polyols prepared in sequence – (a) succinic, (b) adipic, (c) suberic, (d) sebacic acid – each peak corresponded with the prepared polyol structures and positions of protons are denoted by alphabetic characters for better understanding the structure of the capped polyester polyols. As a requirement for more consistent data, 1H-NMR spectrum of each intermediate product was also obtained. 1H-NMR spectra for the first intermediate product, i.e. carboxyl terminated polyester, and second intermediate product, i.e. modified polyester, are shown in Fig. S1 and S2 (ESI†). 1H-NMR spectra from Fig. 3 and S2† were compared to determine the side branches introduced by propionic acid in the final polyester. Peak observed at 1 ppm in the 1H-NMR spectrum of the final polyester was assigned to terminal –CH3 groups. The peak for terminal –CH3 is absent in the 1H-NMR spectrum of the second intermediate product showing that terminal –CH3 groups were absent as shown in Fig. S2.† The overall results indicated that the presence of terminal –CH3 may be due to introduction of side branches as a part of propionic acid. Presence of non-reactive –CH3 groups in replacement of –OH groups can reduce consumption of isocyanate component and reduce the rigid segment of urethane, which matched with our requirements.
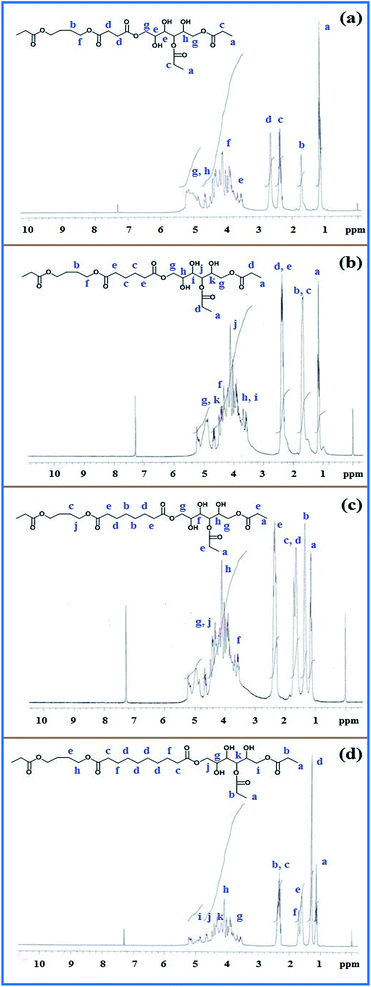 | ||
| Fig. 3 1H-NMR spectra of polyester polyols prepared with the sequence (a) succinic, (b) adipic, (c) suberic, (d) sebacic acid. | ||
Molecular weight
The comparative differences in molecular weights (Mw) of different formulations are represented in Fig. S3 (ESI†), by means of a schematic diagram, which shows the gel permeation chromatographic curve for polyester polyol, and also summarized in Table S3 (ESI†). Mw of polyester polyols was found in the range of 2582–3462, which is substantially high and hence can be increase flexibility to whole moiety. Simultaneously in the series from succinic acid to sebacic acid, the shape of GPC curves changed from narrow to broad molecular weight distribution.Thermal analysis
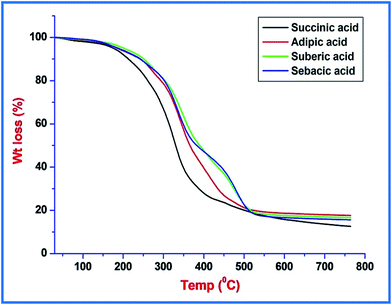
It has been found that, in agreement with the literature, there is a multistep degradation of PU samples as shown in Fig. 4. It is also shown that those dicarboxylic acids used in the polyol part do not influence the basic mechanisms of thermal degradation in the prepared bio-based PU. The shape of the TGA curves remains unchanged even with a change in the dicarboxylic acids due to a very minute difference in chain length of acids.Initial weight loss observed in TGA curves up to a temperature of 120 °C was about 1.797, 1.507, 1.099, and 0.897% for succinic acid, adipic acid, suberic acid, and sebacic acid based formulations, respectively, due to moisture or solvent entrapped in the prepared coatings. Remaining part of the TGA curve of each formulation showed thermal degradation in three major successive steps consisting of urethane bond breaking along with degradation of ester moiety, and aliphatic hydrocarbon chains.33,34 Thermal degradation observed in the first step was associated with internal minute steps. This was observed in the temperature range of 229–269, 221–287, 223–290, and 214–279 °C with weight loss of 9.45, 11.01, 10.51, and 8.13% for PU coatings based on succinic acid, adipic acid, suberic acid, and sebacic acid formulations, respectively, accounting for bond breaking of the urethane moiety. In the next part of the TGA curves, thermal degradation was observed for breakdown of ester moieties and linear hydrocarbon chains in the temperature range 290–590 °C with weight loss of about 62.02–67.97%. In general, among all samples, the PU formulated with the succinic acid based polyester polyol showed the highest thermal stability. The reason for the higher thermal stability of the succinic acid based PU may be due to more cross-linking as it possessed the highest hydroxyl value and thus required more energy to degrade.
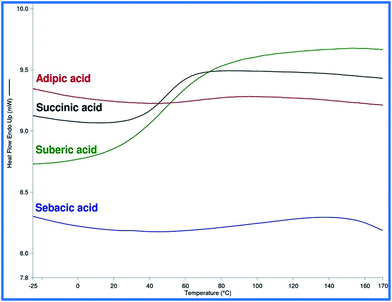
In support of aforesaid thermal degradation data, DSC curves were obtained as shown in Fig. 5. The Tg values for the formulations based on succinic, adipic, suberic, and sebacic acids were found near 48.71, 67.06, 50, and 90.77 °C, respectively. Except for the adipic acid based formulation, the others showed increasing value of Tg with increasing number of C atoms in their backbones.
Coating properties
The coating properties tested for the prepared PU samples included gloss, bending test, adhesion test, pencil hardness as well as mar resistance. All the prepared coating samples showed excellent flexibility, adhesion properties, and pencil hardness. This may be due to the introduction of long aliphatic chain from the difunctional acids as well as the stoichiometric ratio of soft moiety to rigid moiety, which plays a key role in the realization of superior properties. All the formulations have high resistance for pencil hardness parameter and good gloss property. It was observed that as the length of the difunctional acid increased, the pencil hardness and gloss values also increased due to the presence of an increasing number of soft methylene units from succinic to sebacic acid formulations. The mar resistance property showed an increase in the sequence from succinic acid to sebacic acid; however, the values for suberic acid and sebacic acid were found to be the same. This was because both formulations passed the 800 g load successfully but failed for the 1000 g load. This observation revealed that the strength for mar resistance may be within the range of 800–1000 g load for both formulations in sequence. The results are summarised in Table S4 (ESI†).Chemical and solvent resistances
A summary of effects of exposure to selected chemicals including acid (mild and strong) and alkali (mild and strong) along with solvent on formulated two pack PU coating systems prepared from renewable sources and difunctional acids is shown in Table 1. From the table it is clear that exposure to alkali has a tendency to reduce the adhesion property which is the same for all the formulations, i.e. lifting. The most sustainable and less affected formulations among them all, with nearly the same results, are suberic and sebacic acid based resins, which may be because of the presence of higher length of –CH2–.| Chemical/PU coatings | Succinic acid | Adipic acid | Suberic acid | Sebacic acid |
|---|---|---|---|---|
| H2SO4 5% | Lifting | Lifting | Side lifting | Softening |
| HCl 5% | Softening | Lifting | No effect | Lifting |
| CH3COOH 5% | Slight discoloration | Lifting | No effect | Side blistering |
| NaHCO3 5% | Lifting | Lifting | Lifting | Lifting |
| NaOH 5% | Lifting | Lifting | Lifting | Lifting |
| Xylene | Slight discoloration | No effect | No effect | No effect |
Corrosion test
The formulated coatings were produced with film thickness within the range of 50–55 μm. It was found that the PU coating samples containing sebacic acid showed the highest resistance against corrosion among all formulations as shown in Fig. 6 along with Fig. S4 and S5 (ESI†) representing the appearance of coated panels before and after 72 h of immersion, respectively. The formulation with suberic acid also has nearly the same resistance, i.e. renewable formulation has higher resistance rather than the non-renewable adipic acid based formulation. This is because of the higher molecular weight and longer chain length of the sebacic acid based formulation containing more methylene units. Anticorrosive property increased as the formulations go through the sequence from adipic acid to sebacic acid; however, PU from succinic acid showed a problem of paint defect named blistering due to the low molecular weight of the formulation with less resistance.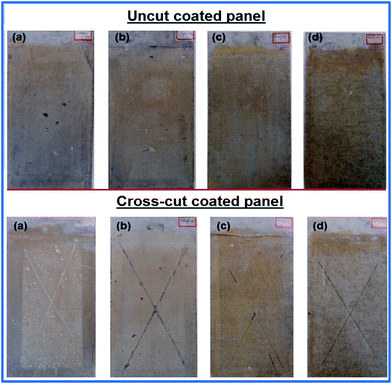 | ||
| Fig. 6 Corrosion showing on coated plates after 168 h for the sequence (a) succinic, (b) adipic, (c) suberic, (d) sebacic acid. | ||
Electrochemical study
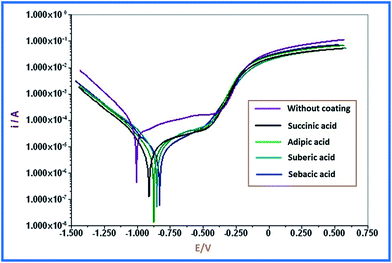
The corrosion resistances for all the PU coatings were assessed by the technique of potentiodynamic polarization, which is universally used for this purpose.35 The Tafel curves were obtained by scanning the potential from a value of −1 V to +1 V above the corrosion potential with a scan rate of 10 mV s−1 to obtain the Tafel polarization measurements for electrochemical analysis of the prepared PU samples. Two types of coated panels were monitored for corrosion rate (CR), corrosion potential (Ecorr), and corrosion current density (Icorr). The panels consisted of (a) without any coating and (b) PU coatings of different formulations. Final results are summarised in Fig. 7 and Table S5.†The blank panel had the lowest anticorrosion property because of the absence of additional media to prevent corrosion, i.e. occurrence of direct contact of coated panel with salt solution, while the results revealed a positive performance of all the coating media as compared to the panel without coating.
In the sequence of formulation series of succinic acid to sebacic acid, the Icorr value was found to decrease, while the Ecorr value increased. Simultaneously, CR decreased for the PU coatings in the series. The relation obtained between CR and Ecorr/Icorr was as per previous observations.36 The results of the electrochemical study indicated that the sebacic acid based formulation was more susceptible with least corrosion. Despite the variation in CR, Ecorr, and Icorr values of the prepared renewable source based PU coatings, the difference among all the formulations was very minute.
Finally, the sebacic acid based formulation was found to have the highest anticorrosive property while the formulation based on succinic acid had the lowest resistance.
Conclusions
Sorbitol and sequential difunctional acid based two pack (2K) PU coatings were prepared successfully and characterized by appropriate techniques. The wide scope of selection for raw materials made it possible to provide a wide range of significant properties in respect of each formulation and revealed satisfactorily results. Whatever the case, any of the formulations can be used separately for coating purpose, but the overall conclusion in regards of comparison was that positive results pointed towards the specific moiety which contains a higher chain length in its chemical structure. It was concluded that the sebacic acid based formulation is the best among all resin formulations on the basis of properties of gloss, pencil hardness, corrosion resistance, etc. That formulation is the most sustainable, suitable, and durable for coating application regarding physical and chemical properties. This indicates favourable structure and property of the sebacic acid based formulation as the most prominent formulation.Acknowledgements
The authors would like to acknowledge the University Grants Commission (UGC), Govt of India, New Delhi for their financial support of Mr Abhijeet Anand in the form of a sanctioned BSR-RFSMS fellowship for research work on the said topic.References
- E. Oledzka and S. S. Narine, J. Appl. Polym. Sci., 2011, 119, 1873 CrossRef CAS.
- M. Alam, A. R. Ray, S. M. Ashraf and S. Ahmad, J. Am. Oil Chem. Soc., 2009, 86, 573 CrossRef CAS.
- P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538 RSC.
- A. Nishio, A. Mochizuki, J. Sugiyama, K. Takeuchi, M. Asai, K. Yonetake and M. Ueda, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 2106 CrossRef CAS.
- B. Ochiai and T. Utsuno, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 525 CrossRef CAS.
- S. Hu and Y. Li, Ind. Crops Prod., 2014, 57, 188 CrossRef CAS.
- A. Chaudhari, A. Kuwar, P. Mahulikar, D. Hundiwale, R. Kulkarni and V. Gite, RSC Adv., 2014, 4, 17866 RSC.
- S. D. Rajput, P. P. Mahulikar and V. V. Gite, Prog. Org. Coat., 2014, 77, 38 CrossRef CAS.
- P. D. Meshram, R. G. Puri, A. L. Patil and V. V. Gite, Prog. Org. Coat., 2013, 76, 1144 CrossRef CAS.
- R. Marin, A. Alla, A. M. Ilarduya and S. Munoz-Guerra, J. Appl. Polym. Sci., 2012, 123, 986 CrossRef CAS.
- J. Haveren, E. A. Oostveen, F. Micciche, B. A. J. Noordover, C. E. Koning, R. A. T. M. van Benthem, A. E. Frissen and J. G. J. Weijnen, J. Coat. Technol. Res., 2007, 4, 177 CrossRef.
- P. Gallezot, Top. Catal., 2010, 53, 1209 CrossRef CAS.
- E. Del Rio, G. Lligadas, J. C. Ronda, M. Galia and V. Cadiz, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 5009 CrossRef CAS.
- Z. S. Petrovic, Polym. Rev., 2008, 48, 109 CrossRef CAS.
- M. S. Gaikwad, V. V. Gite, P. P. Mahulikar, D. G. Hundiwale and O. S. Yemul, Prog. Org. Coat., 2015, 86, 164 CrossRef CAS.
- Y. Xia and R. C. Larock, Green Chem., 2010, 12, 1893 RSC.
- A. B. Chaudhari, A. Anand, S. D. Rajput, R. D. Kulkarni and V. V. Gite, Prog. Org. Coat., 2013, 76, 1779 CrossRef CAS.
- K. Hashimoto, N. Hashimoto, T. Kamaya, J. Yoshioka and H. Okawa, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 976 CrossRef CAS.
- B. Begines, F. Zamora, E. Benito, M. de G. Garcıa-Martın and J. A. Galbis, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 4638 CrossRef CAS.
- S. Rengasamy and V. Mannari, J. Appl. Polym. Sci., 2013, 130, 3874 CAS.
- H. Deka and N. Karak, Prog. Org. Coat., 2009, 66, 192 CrossRef CAS.
- S. D. Rajput, V. V. Gite, P. P. Mahulikar, V. R. Thamke, K. M. Kodam and A. S. Kuwar, J. Am. Oil Chem. Soc., 2014, 91, 1055 CrossRef CAS.
- D. K. Chattopadhyay and K. V. S. N. Raju, Prog. Polym. Sci., 2007, 32, 352 CrossRef CAS.
- A. Anand, R. D. Kulkarni and V. V. Gite, Prog. Org. Coat., 2012, 74, 767 CrossRef.
- H. Kobayashi and A. Fukuoka, Green Chem., 2013, 15, 1740 RSC.
- M. J. Climent, A. Corma and S. Iborra, Green Chem., 2011, 13, 520 RSC.
- P. Gong and L. Zhang, Ind. Eng. Chem. Res., 1998, 37, 2681 CrossRef CAS.
- W. L. Chang, US Pat., US 6,420,446B1, 2002.
- J. M. Raqueza, M. Deléglisea, M. F. Lacrampea and P. Krawczak, Prog. Polym. Sci., 2010, 35, 487 CrossRef.
- C. Bueno-Ferrer, E. Hablot, M. C. Garrigos, S. Bocchini, L. Averous and A. Jiménez, Polym. Degrad. Stab., 2012, 10, 1964 CrossRef.
- V. V. Gite, P. P. Mahulikar, D. G. Hundiwale and U. R. Kapadi, J. Sci. Ind. Res., 2004, 63, 348 CAS.
- B. A. J. Noordover, V. G. Staalduinen, R. Duchateau, C. E. Koning, R. van Benthem, M. Mak, A. Heise, A. E. Frissen and J. van Haveren, Biomacromolecules, 2006, 7, 3406 CrossRef CAS PubMed.
- C. Zhang, Y. Xia, R. Chen, S. Huh, P. A. Johnston and M. R. Kessler, Green Chem., 2013, 15, 1477 RSC.
- H. Sui, X. Ju, X. Liu, K. Cheng, Y. Luo and F. Zhong, Polym. Degrad. Stab., 2014, 101, 109 CrossRef CAS.
- X. X. Yan and G. Y. Xu, J. Alloys Compd., 2010, 491, 649 CrossRef CAS.
- Y. Cheng and Y. F. Zheng, Mater. Sci. Eng., A, 2006, 438–440, 1146 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra17202k |
| This journal is © The Royal Society of Chemistry 2016 |