Enzymatically crosslinked epsilon-poly-L-lysine hydrogels with inherent antibacterial properties for wound infection prevention†
Rui Wanga,
De-lei Xua,
Lei Liangb,
Ting-ting Xua,
Wei Liua,
Ping-kai Ouyanga,
Bo Chi*a and
Hong Xu*a
aState Key Laboratory of Materials-Oriented Chemical Engineering, Nanjing Tech University, No. 30 South Puzhu Road, Pukou District, Nanjing 211816, China. E-mail: chibo@njtech.edu.cn; xuh@njtech.edu.cn
bDepartment of Comparative Medicine, Nanjing General Hospital of Nanjing Military Command, No. 305 Zhongshan East Road, Nanjing 210002, China
First published on 5th January 2016
Abstract
In this study, in situ forming epsilon-poly-L-lysine (EPL) bioadhesive hydrogels were fabricated for wound infection prevention. The hydrogel precursor polymer consisted of EPL backbone conjugated with phenol groups of hydroxyphenylpropionic acid (HPA). The chemical modification was characterized by 1H NMR and UV spectroscopies. An enzymatic crosslinking method was employed to copolymerize EPL-HPA (EHPA) in the presence of horseradish peroxidase (HRP) and hydrogen peroxide (H2O2). The HRP crosslinking system allows rapid gelation within several seconds. The hydrogel properties, including the gelation rate, the mechanical strength, and the degradation behavior, were adjustable by controlling the concentration of HRP, H2O2, and the polymer. The adhesiveness ranged from 10 kPa to 35 kPa, which is higher than that of fibrin glue. Cytotoxicity assay with L929 fibroblasts revealed that the hydrogels possessed good in vitro biocompatibility. In addition, in vitro antibacterial studies showed that these bioadhesive hydrogels exhibited an impressively wide spectrum of antimicrobial activity against both Gram-negative and Gram-positive bacteria. Furthermore, evaluations of in vivo subcutaneous injection revealed that the hydrogels have a significant in vivo anti-infection effect. These results suggest that the in situ forming EPL-based hydrogels are interesting and promising bioadhesive materials with inherent antibacterial properties for wound infection prevention.
Introduction
Despite intensive research on aseptic techniques and sterilization, wound infection has not been deracinated when associated with surgical procedures. It has been a huge challenge for surgeons in clinical medicine. Many attendant problems, such as complications, medical risks, and even mortality, are often inevitable.1–3 The most common inducements responsible for wound-bed infection are microorganisms, which exist abundantly in the environment and inside the human body.4–6 As a consequence, much effort has been made to prevent surgery-associated infection by loading antimicrobial drugs, such as antibiotic chemicals, into materials for local delivery.7–14 However, antibiotics and antimicrobial chemicals have limitations that arise from their short-term antimicrobial effect, because these materials will eventually lose their antibiotic activity with the active agent being released from the system over time. Also, their toxicity and the possibility of causing microbe resistance are other problems of this class.15,16Consequently, materials with long-term antimicrobial effect and minimal bacterial resistance are highly desirable in surgical practice.
Antimicrobial hydrogels have emerged as promising materials for combating infections. Hydrogels are crosslinked three-dimensional networks of hydrophilic polymers, which are not easy to dissolve under physiological conditions, and so can maintain long-term antimicrobial activity.17 As a type of hydrogel, bioadhesives have received increasing attention as anti-infective material because they can well interface with tissue, which could prevent the potential formation of wound dead space in which microorganisms could breed easily.3 Furthermore, when a bioadhesive hydrogel is functionalized by antimicrobial peptides (AMPs), it is highly advantageous over conventional antibiotic doping, because AMPs exhibit more suitable properties for anti-infection, such as inherent anti-infective ability, broad spectrum activity, and minimal bacterial resistance.18,19
One of the prime candidates, epsilon-poly-L-lysine (EPL), is a homo-poly-amino acid characterized by a peptide bond between the ε-amino and α-carboxyl groups of L-lysine. As an inherently antimicrobial material, EPL possesses broad-spectrum antimicrobial activity against both Gram-negative (such as E. coli) and Gram-positive bacteria (such as S. aureus), as well as fungi (such as Candida albicans).20–24 Although the mechanisms responsible for antimicrobial activity are still debated, it typically involves membrane disruption resulting in cell lysis.25 In addition, EPL has many beneficial properties, such as biocompatibility, biodegradability, and nontoxicity.25–29 These attributes make it ideal for wound infection prevention.
Herein, we describe an in situ antimicrobial bioadhesive hydrogel based on EPL obtained using an enzyme-catalyzed crosslinking approach in the presence of horseradish peroxidase (HRP) and H2O2. The polymerization reaction was achieved via the oxidative coupling of phenol groups or aniline moieties in water-soluble polymers. Unlike chemical gelation, enzyme-triggered reactions proceed under mild conditions, which enable EPL to form a hydrogel in situ for a desired time period and allow the adjustment of physicochemical characteristics, such as gelation rate, mechanical properties and degradation time. Most of all, the hydrogel exhibits excellent antimicrobial ability both in vitro and in vivo, while being biocompatible.
Experimental section
Materials
EPL (MW, 3.5–5 kDa) was obtained from Nanjing Shineking Biotechnology Co. Ltd. 1-Ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC), N-hydroxysuccinimide (NHS), 3,4-hydroxyphenylpropionic acid (HPA), H2O2, and HRP (type VI; 298 purpurogallin U mg−1 solid) were purchased from Sigma-Aldrich. Phosphate buffered saline (PBS, pH 7.4) was purchased from B. Braun Co. All other chemicals and solvents were used without further purification.Synthesis and characterization of EHPA conjugates
EHPA conjugates were prepared by a general carbodiimide/active ester-mediated coupling reaction. In brief, HPA (0.83 g, 5 mmol) was dissolved in 75 mL co-solvent of water and DMF (volume ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). To the solution, EDC (0.955 g, 5 mmol) and NHS (0.8 g, 6.95 mmol) were added, and the solution was stirred at room temperature for 4 h. The activated HPA solution was added to 50 mL EPL solution (9.10 wt%), and the resulting mixture was stirred at room temperature overnight. The reaction mixture was transferred to a dialysis bag with a molecular cut-off of 1000 Da and dialyzed against deionized water for 3 days. The purified solution was filtered and lyophilized to obtain the EHPA conjugates. We determined the structure and composition of EHPA by 1H NMR (400 MHz; Varian, Palo Alto, USA). The phenolic contents of the conjugates were quantitatively measured with an ultraviolet-visible spectrophotometer (JASCO V-570).
2). To the solution, EDC (0.955 g, 5 mmol) and NHS (0.8 g, 6.95 mmol) were added, and the solution was stirred at room temperature for 4 h. The activated HPA solution was added to 50 mL EPL solution (9.10 wt%), and the resulting mixture was stirred at room temperature overnight. The reaction mixture was transferred to a dialysis bag with a molecular cut-off of 1000 Da and dialyzed against deionized water for 3 days. The purified solution was filtered and lyophilized to obtain the EHPA conjugates. We determined the structure and composition of EHPA by 1H NMR (400 MHz; Varian, Palo Alto, USA). The phenolic contents of the conjugates were quantitatively measured with an ultraviolet-visible spectrophotometer (JASCO V-570).
Gelation time and mechanical strength measurement
Hydrogel samples were prepared in vials at room temperature. First, 200 μL of PBS solution (pH 7.4) containing the EHPA polymer (10 wt%) was equally divided into two parts. PBS solutions of H2O2 (100 μL of 0.06 wt% stock solution) and HRP (50 μL of 0.01–0.05 mg mL−1 stock solution) were separately added to each half of the PBS solution with EHPA. Both halves were gently mixed to form the in situ gel (Scheme 1).30 The time to form a gel (gelation time) was defined via the vial tilting method.31 When no flow was observed within 1 min after inverting the vial, the solution was regarded as being in the gel state. The elastic modulus (G′) of the hydrogels was measured with a rheometer (Bohlin Advanced Rheometer; Malvern Instruments) using a parallel plates (20 mm diameter) configuration at 37 °C. The EHPA polymers (2, 5, and 10 wt%) were dissolved in HRP solution (0.05 mg mL−1 of the stock solution) and in different concentrations of H2O2 (0.02–0.06 wt% of the stock solution) solutions. The mixture was vortexed and immediately applied to the bottom plate. The upper plate was lowered to a measurement gap size of 1 mm, and a layer of silicon oil was carefully applied around the plate to prevent solvent evaporation during the experiment. The measurements were taken at 37 °C in the dynamic oscillatory mode with a frequency of 0.1 Hz (single frequency) and a strain of 0.1%.Scanning electron microscopy (SEM)
The microporous morphologies of dehydrated EHPA hydrogels were characterized by SEM. Hydrogel disks were prepared with EHPA solutions (10 wt%) dissolved in HRP solution (0.05 mg mL−1 stock solution) and different H2O2 concentrations (0.02–0.06 wt% of stock solution). Then they were washed in distilled water for 3 days to remove the residual unreacted molecules in the matrix. The hydrogels were then freeze-dried and gold-coated. The cross-sectional morphologies were viewed by SEM (Philips-FEI, Holland) at an accelerating voltage of 3 kV.Study on tissue adhesion using universal testing machine
Adhesion properties were determined by lap shear strength tests with a universal testing machine (Instron model 4466) according to the modified ASTM method F2255-05.32,33 The hydrogels for this test were prepared as described above. Porcine skin was selected as a substrate for the experiment and cut into 3 cm × 1.5 cm pieces. Subsequently, 50 μL of the mixture was dispensed and spread over an area of 1 cm × 1.5 cm of a piece of porcine skin. Another piece of specimen was immediately overlain on the part applied with the adhesive and compressed for 10 min at room temperature. Subsequently, the adhesive strength was measured with a 100 N load cell at a cross-head speed of 1 cm min−1. As a control, fibrin glue was assessed under the same conditions. Each measurement was repeated five times.In vitro degradation test
Hydrogel samples with different H2O2 concentrations were prepared in accordance with the above mentioned procedure and accurately weighed (Wi). The samples were placed in test tubes containing 10 mL of PBS (pH 7.4) and incubated at 37 °C for pre-determined times. At specified time intervals, the buffer solution was removed from the samples and the hydrogels were weighed (Wt). Subsequently, fresh PBS solution was supplemented to the tubes. The weight percentage of the hydrogels was plotted as a function of the incubation time and expressed as Wt/Wi. All samples were tested in triplicate.Cytotoxicity evaluation
The in vitro cytocompatibility of the hydrogel was evaluated by MTT and live/dead assay in accordance with the guidelines of the International Organization for Standardization (ISO) and the local Ethics Committee.34 L929 mouse fibroblasts were selected as the object of the study. For this study, EHPA polymer solution (10 wt%), HRP (0.05 mg mL−1), and H2O2 solution (0.06 wt%) were first sterilized by filtration with 200 nm syringe filters. All the experiments were performed on a sterile ultraclean workbench. The polymer solution (containing HRP) was transferred to a 96-well plate. The L929 mouse fibroblast suspension was added and mixed with the polymer solution. After which, the cell-containing polymer solution was mixed with the same volume of H2O2 solution. The cell-immobilized hydrogels were formed in situ by gently blending the mixture. The density of the cells in the hydrogel was 1 × 106 cells per mL. The hydrogel matrices were cultured for 24, 48, and 72 h in DMEM containing 10% FBS and 1% penicillin/streptomycin in an atmosphere of 5% CO2 at 37 °C. At regulated times, the medium was removed and the hydrogels were rinsed twice with PBS. Subsequently, MTT reagent (methylthiazolydiphenyltetrazolium bromide) together with fresh medium (100 μL) was introduced into each well and incubated for 4 h at 37 °C. The supernatant was transferred to a blank well, and the UV absorbance at 570 nm was read with a microplate reader (Biorad). To observe cell morphology in hydrogels, a solution of LIVE/DEAD staining fluorescence dye (Calcein AM (final concentration, 2 μM) and ethidium homodimer-1 (final concentration, 4 μM)) was added to each well, and the cells were incubated in the dark for 1 h at room temperature. The morphology of fluorescently labeled cells was visualized by laser scanning confocal microscopy (Leica TCS SP5).In vitro antibacterial studies
E. coli (Gram-negative) and S. aureus (Gram-positive) were used as test organisms to evaluate the in vitro antibacterial activity of EHPA hydrogels. Luria–Bertani (LB) agar was employed throughout this experiment as the growth medium. First, 100 mL of liquid LB medium containing 10 g L−1 tryptone, 5 g L−1 yeast extract, 10 g L−1 sodium chloride, and 10 g L−1 agar was prepared and autoclaved for 20 min at 121 °C. After cooling to 60 °C, the medium was poured into Petri dishes, and cooled to harden. Then four circular holes (1 cm in diameter) in symmetrical places of the matrix were removed with an agar punch. About 100 μL of EHPA (10 wt%) bioadhesive hydrogel was used to fill two of the cavities. As a control, the two remaining cavities were filled with agar gel. The bioadhesive was incubated for 15 min at 37 °C. Subsequently, 1 mL of bacteria (108 CFU mL−1) was sprayed on the agar Petri dish to ensure the surfaces of bioadhesive and agar gel were uniformly covered with bacteria. The plates were incubated at 37 °C for 24 h. The inhibitory effect of hydrogels on bacteria was determined quantitatively by measuring the diameters of the bacteria-free zones surrounding the gels. The average value of the two bacteria-free zones was calculated, and that was defined as the diameter of the inhibition zone.For the bacterial morphology study, two pieces of 2 × 2 cm EHPA hydrogel and LB agar gel were prepared as mentioned above. 20 μL bacteria suspension was uniformly spread on the top surface of the gels, followed by incubation at 37 °C for 2 h. Then the bacteria were fixed with glutaraldehyde (2.5%) for 4 h, after which the gel plates were dehydrated using an ethanol series (20–100%; 15–30 min), and subsequently freeze-dried. The dried hydrogels were characterized by SEM (Philips-FEI, Holland) for microbe morphology examination.
In vivo activity of bioadhesive hydrogels
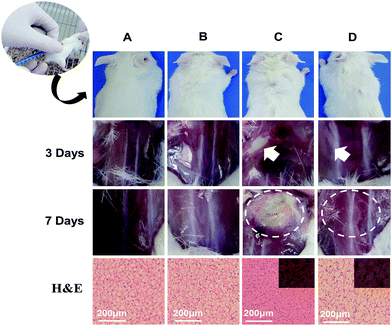
An in vivo animal study of the bioadhesive was conducted with 12 healthy male BALB/c mice (6 to 8 weeks old; Jinling Hospital, Nanjing, China) using the subcutaneous injection of bacteria model.3 All the animal care and experimental protocols were reviewed and approved by the Animal Investigation Ethics Committee of Jinling Hospital. The mice were randomly divided into four groups, and each group contained three mice. Both the EHPA hydrogels and S. aureus (108 CFU mL−1) for this test were prepared as described previously in the section on in vitro antibacterial studies. Briefly, group one was subcutaneously injected with 200 μL of sterile saline in the middle of the dorsal region (control). Group two was injected with 200 μL of EHPA hydrogels in the same manner as group one. Group three was injected with 100 μL of S. aureus alone. Group four was injected with 100 μL of S. aureus, followed by injecting with 200 μL of EHPA hydrogels where the bacteria exist. At day 3, infection was apparent in the animals of group three. Half of the animals in each group were killed and the infection site was dissected. At 7 days post-infection, all the remaining animals were killed and the infection site was dissected. Meanwhile, the tissue of the injection site was excised, fixed with 10% formalin, and stained with hematoxylin–eosin (H&E) reagent for histological observations.Results and discussion
Synthesis and characterization of EHPA
EHPA conjugates were prepared by a general carbodiimide/active ester-mediated coupling reaction in a co-solvent of water and DMF, as shown in Fig. 1a. From the 1H NMR spectra of EHPA, the peaks at chemical shifts of 6.8 ppm and 7.1 ppm indicated the presence of aromatic protons of HPA (Fig. 1b). The degree of substitution (DS) of HPA was calculated from the ratio of the integrated areas of peak 8 and 9 to peak 4 to be 11% (ESI, Fig. S1†). This indicates that about 90% excess free amino groups of EPL still exist in the EHPA conjugates. These remaining free amino groups provide the possibility for further antibacterial activity application. The HPA content in the EHPA conjugates was determined by UV measurements (at 275 nm) (Fig. 1c) and calculated to be ∼160 μmol g−1 EHPA conjugate (data not shown).Preparation of in situ hydrogels and gelation time
Various chemical gelation reactions have been previously induced by agents such as carbodiimides or glutaraldehyde.35 However, these agents are difficult to apply in vivo because of the toxicity of these crosslinking agents.36 In the present study, the EHPA hydrogels were prepared by enzymatic crosslinking. The EHPA hydrogels were prepared by blending the prefabricated polymer, enzyme solutions, and H2O2. Enzymatic crosslinking of EHPA solutions allowed the phenol moieties to couple to each other either through C–C bonds between ortho-carbons of the aromatic ring or through C–O bonds between ortho-carbons and phenolic oxygen (Fig. 2a).4,37 Rapid in situ gelation is essential to quickly cover a wound surface and prevent infection in a surgical site. However, very rapid gelation is detrimental to applications in vivo because of there being insufficient time for tissue manipulation after the adhesive has been administered. In addition, very rapid gelation can result in non-homogeneous hydrogels. In our study, the gelation time of the EHPA hydrogels could be adjusted by changing the concentrations of the polymer and enzymes. Transparent and homogeneous hydrogels were formed under mild conditions. The gelation time of the EHPA hydrogels was investigated at various HRP concentrations (Fig. 2b). It was observed that the gelation time decreased with increasing concentration of HRP from 0.01 mg mL−1 to 0.05 mg mL−1 when the contents of EHPA copolymer and H2O2 were fixed at 10 wt% and 0.06 wt%, respectively. This phenomenon could be explained in that higher concentrations of HRP led to more generated phenoxyl radicals, which reacted with hydroxyl groups or ortho-carbons of phenol groups in EHPA to accelerate the enzymatic coupling reaction. Furthermore, note that the gelation was faster with higher EHPA concentrations because the higher phenolic content could provide a larger number of electron acceptors and oxidized donors in the enzymatic reaction.38Rheological studies
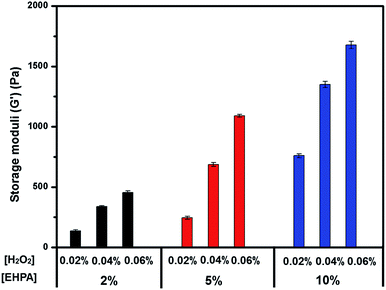
Oscillatory rheology experiments were used to study the mechanical properties of the EHPA hydrogels with different concentrations of polymer solutions (2, 5, and 10 wt%) and H2O2 (0.02–0.06 wt% of the stock solution), which contained a fixed HRP content (0.05 mg mL−1) in PBS at 37 °C. A mixture of HRP and H2O2 solutions and a solution of EHPA were applied to the rheometer with a double syringe equipped with a mixing chamber. The storage modulus rapidly increased after mixing the reactants because of the high-efficiency enzymatic crosslinking reactions. The plateau values (G′) are defined as the endpoints of the crosslinking reaction. As shown in Fig. 3, a higher polymer concentration generally resulted in a higher storage modulus of the hydrogels. Moreover, the elastic modulus of the hydrogels could be adjusted by changing the H2O2 concentration. For example, increasing the H2O2 concentration led to higher G′ values when the concentrations of HRP and EHPA were fixed at 10 wt% and 0.05 mg mL−1, respectively. This result was associated with increased phenoxy radical generation, thereby leading to increased crosslinking density during HRP-catalyzed reactions.Morphology of dehydrated hydrogels
The microstructural morphologies of the EHPA hydrogels formed with different concentrations of H2O2 are shown in Fig. 4. The samples were prepared as previously mentioned. The concentrations of the EHPA polymer, H2O2, and HRP used in the formation of the EHPA hydrogels are listed in Table 1 (ESI†). As expected, the pore size of the freeze-dried hydrogels decreased with increasing H2O2 concentration. Fig. 4 shows that a higher ratio of H2O2 resulted in smaller pore sizes. This trend was attributed to the increased H2O2 content, which resulted in an increased crosslinking density in the hydrogel, thereby supporting the rheological results. This result further demonstrated that the mechanical strength could be regulated by the H2O2 concentration.Degradation behavior of EHPA hydrogels
The in vitro degradation assay of EHPA hydrogels was performed with different concentrations of H2O2. The hydrogels used in this experiment were prepared as previously described and incubated in 0.01 M PBS at 37 °C for predetermined time intervals. As shown in Fig. 5, the hydrogels with higher H2O2 concentrations showed a slower weight loss rate than hydrogels with lower H2O2 concentrations. For instance, the hydrogels exhibited the highest swelling ratio and lowest weight loss when the highest concentration of H2O2 (0.06 wt%, EHPA-C) was used. However, when H2O2 was used at the lowest concentration (0.02 wt%, EHPA-A), the swelling ratio was almost negligible with the highest weight loss. The slower degradation of EHPA hydrogels at a higher H2O2 concentration could be attributed to the higher crosslinking densities in the hydrogels, which was also demonstrated by the microstructural morphologies. Generally, the EHPA hydrogels were completely degraded after a month. Therefore, it is concluded that the degradation rates of the hydrogels depend on their crosslinking density. Moreover, these results indicated that the degradation time of the EHPA hydrogels could be easily modulated by changing the H2O2 content in the formed hydrogels.Tissue adhesive strength
The adhesive strength of the EHPA hydrogels was evaluated at different H2O2 concentrations. The hydrogels were prepared using the same conditions as mentioned above. Porcine skins were used as the substrate materials, and fibrin glue was used as the control bioadhesive. As shown in Fig. 6, the adhesiveness of fibrin glue was about 5 kPa, which was in line with the results of previous reports in the range of 0.5–10 kPa.39 By contrast, EHPA hydrogels revealed higher adhesiveness, which ranged from 10 kPa to 35 kPa. Although the intrinsic nature of the mechanism related to tissue–material adhesion at the molecular level remains unclear, it has been reported that cationic polymers can interact with tight junctions in epithelial cells followed by generation of an invertible opening and reorientation of the tight junction.40,41 The adhesive properties of EHPA hydrogels were mainly attributed to the interaction between the positively charged amino groups of EPL with negatively charged sialic acid groups on the mucus membrane.42–44 Meanwhile, Fig. 6 shows that the adhesion of the hydrogels increased with increasing H2O2 concentration. This phenomenon may originate from different network structures of the hydrogels. As indicated in previous studies, a higher crosslinking density can often enhance the adhesive strength of polymers because a tighter gel network can provide stronger cohesive interactions to help resist bond failure.45Cytocompatibility of EHPA hydrogels
To evaluate the cytocompatibility of the EHPA hydrogels, cells were cultured within the hydrogel matrices for 3 days. Fibroblast cells were employed to evaluate cytotoxicity because they play a crucial role in wound healing and constitute part of the connective tissues, which is a prospective application of functionalized hydrogels.46,47 Cell viability was determined by a LIVE–DEAD assay kit as a function of the culture time. The viable (stained green) and dead (stained red) cells were monitored by fluorescence microscopy. Notably, a majority of the cells in all hydrogels were viable even after 3 days of culture, and no dead cells were detected (Fig. 7a). These results indicated that the hydrogels exhibited excellent cytocompatibility. MTT assay was used to confirm these findings. As shown in Fig. 7b, at 24 h of incubation, the cell viability of the hydrogel cultures was lower than that of the normal culture plates (control). However, at 48 h of incubation, no statistically significant differences were observed between them, and cell viabilities were higher than those of the control after 72 h of incubation. The obtained results clearly suggested that EHPA hydrogels are nontoxic to L929 cells, and they are good candidates for use as bioadhesives.In vitro antibacterial properties
The in vitro antibacterial activity was evaluated against E. coli and S. Aureus by visual inhibition zone tests. As shown in Fig. 8a, the inhibition zone was obviously noticeable in the interface between the EHPA hydrogels and agar for both strains. This suggested that the EHPA hydrogels completely inhibit growth of E. coli and S. aureus. By contrast, the agar gel did not show any toxicity towards both strains. The quantified results of inhibition zone tests are shown in ESI, Fig. S2.† The efficient antibacterial activity could be due to the presence of EPL in the hydrogel system. It has been reported that EPL kills microorganisms by adsorbing onto the microbe surface followed by damaging the outer membrane of the cell, which leads to cell wall/membrane lysis.25 To further investigate the antimicrobial mechanism, we used a SEM observation method based on the morphological changes of microbes. Fig. 8b shows that cells in contact with the control gel retained their natural rodlike (E. coli) and round (S. aureus) shapes. Conversely, those treated with EHPA hydrogels exhibit apparent distorted and withered surfaces. This phenomenon agrees with the antimicrobial mechanism of EPL.In vivo evaluation
To demonstrate the clinical potential of the antimicrobial gels, the EHPA hydrogels were further studied in vivo by subcutaneously injecting into mice with bacteria. S. aureus, one of the most common bacteria responsible for wound infection,4 was used for this test. The mice were treated with sterile saline, hydrogels, S. aureus, and hybrid of S. aureus and hydrogels, respectively. At day 3, the positive control mice treated with S. aureus were close to death, thereby suggesting clinically evident infection. Half of experimental animals were killed and dissected at the infection site. Fig. 9 shows an obvious abscess in the S. aureus treated mice. In sharp contrast, when the animals were injected with EPHA hydrogels together with S. aureus, the infection did not happen. The morphology of internal tissue at the injection site was just like the control animals treated with sterile saline. Also, the group treated with hydrogel alone was not infected. This is consistent with the hydrogels' good biocompatibility. After one week, all the remaining animals were sacrificed and the tissue samples were harvested and analyzed by H&E staining. As shown in Fig. 9, a high level of infection can be clearly observed in the S. aureus treated mice. In addition, the histology image shows the apparent inflammatory response. A large amount of inflammatory cell infiltration was detected at the site of the bacterial injection. In contrast, when S. aureus was injected together with the bioadhesive, no active infection was observed, which was similar to the healthy control mice treated with sterile saline and bioadhesive alone. These results clearly indicate the outstanding anti-infection property of the EHPA hydrogels.Conclusions
In summary, injectable bioadhesive hydrogels based on EPL were successfully fabricated in situ by enzymatic crosslinking via HRP/H2O2 and then carefully characterized in this study. The bioadhesive showed adjustable mechanical properties and good adhesive strengths. In addition, the cytotoxicity experiment showed that the prepared bioadhesive had excellent biocompatibility. Importantly, these bioadhesives are inherently antibacterial and kill Gram-positive and Gram-negative bacteria. Overall, our results suggested that EPL-based hydrogels have great potential for combating infection in surgical sites and for other medical applications.Acknowledgements
This work was supported by the National Research Nature Science Foundation of China (no. 21176123, 31401588 and 51403103), the National Basic Research Program of China (no. 2013CB733603), and the Research Nature Science Foundation of Jiangsu Province (no. BK2012428 and 11KJB150005), State Key Laboratory of Materials-Oriented Chemical Engineering KL14-01 and KL12-02.Notes and references
- E. M. Hetrick and M. H. Schoenfisch, Chem. Soc. Rev., 2006, 35, 780–789 RSC.
- C. D. Owens and K. Stoessel, J. Hosp. Infect., 2008, 70, 3–10 CrossRef PubMed.
- C. G. Michael, I. Zuhaib, H. M. Scott, A. S. Karim, M. C. Joani, Y. Yuji, B. Gerald and P. S. Joel, Nat. Commun., 2014, 5, 4095 Search PubMed.
- K. P. T. Sudheesh, V. K. Lakshmanan, T. V. Anilkumar, C. Ramya, P. Reshmi, A. G. Unnikrishnan, V. N. Shantikumar and R. Jayakumar, ACS Appl. Mater. Interfaces, 2012, 4, 2618–2629 Search PubMed.
- R. P. Wenzel and T. M. Perl, J. Hosp. Infect., 1995, 31, 13–24 CrossRef CAS PubMed.
- L. A. Gordon, Clin. Infect. Dis., 1998, 26, 1179–1181 Search PubMed.
- B. G. Elliott, N. P. Theodore, A. K. Walter, W. F. James, L. Min, G. Jyotsna, B. M. Daniel, E. M. Feza, H. R. Virgilio, V. G. Kerstin and N. R. N. Corey, N. Engl. J. Med., 2010, 363, 1038–1049 CrossRef PubMed.
- A. Hui, H. Sheardown and L. Jones, Materials, 2012, 5, 85–107 CrossRef CAS.
- E. D. Giglioa, S. Cometab, M. A. Riccia, D. Cafagnaa, A. M. Savinoa, L. Sabbatinia, M. Orcianic, E. Cecid, L. Novellod, G. M. Tantillod and B. M. Mattioli, Acta Biomater., 2011, 7, 882–891 CrossRef PubMed.
- G. Laverty, S. P. Gorman and B. F. Gilmore, J. Biomed. Mater. Res., Part A, 2012, 100, 1803–1814 CrossRef PubMed.
- S. B. Goodman, Z. Yao, M. Keeney and F. Yang, Biomaterials, 2013, 34, 3174–3183 CrossRef CAS PubMed.
- K. D. Sinclair, T. X. Pham, R. W. Farnsworth, D. L. Williams, C. Loc-Carrillo, L. A. Horne, S. H. Ingebretsen and R. D. Bloebaum, J. Biomed. Mater. Res., Part A, 2012, 100, 2732–2738 CrossRef CAS PubMed.
- M. Kazemzadeh-Narbat, S. Noordin, B. A. Masri, D. S. Garbuz, C. P. Duncan, R. E. W. Hancock and R. Wang, J. Biomed. Mater. Res., Part B, 2012, 100, 1344–1352 CrossRef PubMed.
- K. G. Kristinsson, B. Jansen, U. Treitz, P. F. Schumacher, G. Peters and G. Pulverer, J. Biomater. Appl., 1991, 5, 173–184 CrossRef CAS PubMed.
- A. W. Smith, Adv. Drug Delivery Rev., 2005, 57, 1539–1550 CrossRef CAS PubMed.
- M. Zasloff, Nature, 2002, 415, 389–395 CrossRef CAS PubMed.
- Y. Li, K. Fukushima, D. J. Coady, A. C. Engler, S. Q. Liu, Y. Huang, J. S. Cho, Y. Guo, L. S. Miller, J. P. K. Tan, P. L. R. Ee, W. M. Fan, Y. Y. Yang and J. L. Hedrick, Angew. Chem., Int. Ed., 2013, 52, 674–678 CrossRef CAS PubMed.
- A. Cherkasov, K. Hilpert, H. Jenssen, C. D. Fjell, M. Waldbrook, S. C. Mullaly, R. Volkmer and R. E. W. Hancock, Biology, 2009, 4, 65–74 CAS.
- M. Gabriel, K. Nazmi, E. C. Veerman, A. V. N. Amerongen and A. Zentner, Bioconjugate Chem., 2006, 17, 548–550 CrossRef CAS PubMed.
- J. Hiraki, Fine Chemicals, 2000, 29, 25–28 Search PubMed.
- N. Delihas, L. W. Riley, W. Loo, J. Berkowitz and N. Poltoratskaia, FEMS Microbiol. Lett., 1995, 132, 233–237 CrossRef CAS PubMed.
- H. Y. Ting, J. Tokyo Univ. Fish., 1997, 84, 25–30 Search PubMed.
- M. Kito, Y. Onji, T. Yoshida and T. Nagasawa, FEMS Microbiol. Lett., 2002, 207, 147–151 CAS.
- S. Shima and H. Sakai, Agric. Biol. Chem., 1981, 45, 2503–2508 CrossRef CAS.
- S. Shima, H. Matsuoka, T. Iwamoto and H. Sakai, J. Antibiot., 1984, 37, 1449–1455 CrossRef CAS PubMed.
- J. Farrera-Sinfreu, E. Giralt, S. Castel, F. Albericio and M. Royo, J. Am. Chem. Soc., 2005, 127, 9459–9468 CrossRef CAS PubMed.
- P. H. Mygind, R. L. Fischer, K. M. Schnorr, M. T. Hansen, C. P. Sonksen and S. Ludvigsen, Nature, 2005, 437, 975–980 CrossRef CAS PubMed.
- R. Rathinakumar, W. F. Walkenhorst and W. C. Wimley, J. Am. Chem. Soc., 2009, 131, 7609–7617 CrossRef CAS PubMed.
- R. Wang, B. Zhou, W. Liu, X. H. Feng, S. Li, D. F. Yu, J. C. Chang, B. Chi and H. Xu, J. Biomater. Appl., 2015, 29, 1167–1179 CrossRef CAS PubMed.
- J. X. Hou, C. Li, Y. Guan, Y. J. Zhang and X. X. Zhu, Polym. Chem., 2015, 6, 2204–2213 RSC.
- S. Kull, I. Martinelli, E. Briganti, P. Losi, D. Spiller and S. Tonlorenzi, J. Surg. Res., 2009, 157, 15–21 CrossRef PubMed.
- L. Ninan, J. Monahan, R. L. Stroshine, J. J. Wilker and R. Shi, Biomaterials, 2003, 24, 4091–4099 CrossRef CAS PubMed.
- E. S. Mohammed, E. S. Martha and F. B. Helge, Anal. Bioanal. Chem., 2005, 381, 557–567 CrossRef PubMed.
- K. Y. Lee and D. J. Mooney, Chem. Rev., 2001, 101, 1869–1879 CrossRef CAS PubMed.
- S. Sakai, K. Hirose, K. Taguchi, Y. Ogushi and K. Kawakami, Biomaterials, 2009, 30, 3371–3377 CrossRef CAS PubMed.
- L. S. Wang, J. E. Chung, P. P. Chan and M. Kurisawa, Biomaterials, 2010, 31, 1148–1157 CrossRef CAS PubMed.
- (a) R. Jin, L. S. Teixeira, P. J. Dijkstra, C. A. Blitterswijk, M. Karperien and J. Feijen, Biomaterials, 2010, 31, 3103–3113 CrossRef CAS PubMed; (b) S. Sakai, K. Hirose, K. Moriyama and K. Kawakami, Acta Biomater., 2010, 6, 1446–1452 CrossRef CAS PubMed.
- (a) S. A. Burke, M. R. Jones, B. P. Lee and P. B. Messersmith, Biomed. Mater., 2007, 2, 203 CrossRef CAS PubMed; (b) I. Strehin, Z. Nahas, K. Arora, T. Nguyen and J. A. Elisseeff, Biomaterials, 2010, 31, 2788–2795 CrossRef CAS PubMed.
- A. A. Ibrahim, Int. J. Biol. Macromol., 2009, 45, 16–21 CrossRef PubMed.
- J. Smith, E. Wood and M. Dornish, Pharm. Res., 2004, 21, 43–49 CrossRef CAS.
- N. Nakajima, H. Sugai, S. Tsutsumi and S. H. Hyon, Key Eng. Mater., 2007, 342, 713–716 CrossRef.
- W. Nie, X. Y. Yuan, J. Zhao, Y. L. Zhou and H. J. Bao, Carbohydr. Polym., 2013, 96, 342–348 CrossRef CAS PubMed.
- Y. L. Zhou, W. Nie, J. Zhao and X. Y. Yuan, J. Mater. Sci.: Mater. Med., 2013, 24, 2277–2286 CrossRef CAS PubMed.
- C. R. Matos-Pérez, J. D. White and J. J. Wilker, J. Am. Chem. Soc., 2012, 134, 9498–9505 CrossRef PubMed.
- R. Shrivastava, Diabetes Res. Clin. Pract., 2011, 92, 92–99 CrossRef PubMed.
- C. Z. Wu, C. Strehmel, K. Achazi, L. Chiappisi, J. Dernedde, M. C. Lensen, M. Gradzielski, M. B. Schumacher and R. Haag, Biomacromolecules, 2014, 15, 3881–3890 CrossRef CAS PubMed.
- S. Venkataraman, Y. Zhang, L. H. Liu and Y. Y. Yang, Biomaterials, 2010, 31, 1751–1756 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra15616e |
| This journal is © The Royal Society of Chemistry 2016 |