Distribution of carbon nanotubes in fresh ordinary Portland cement pastes: understanding from a two-phase perspective†
Abstract
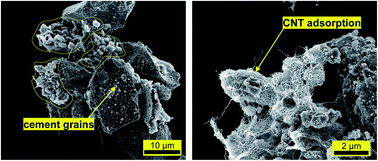
Significant research advances have been made in the field of carbon nanotube (CNT) reinforced ordinary Portland cement (OPC) paste composites in recent years. However, the distribution of CNTs in fresh OPC paste is yet to be fully researched and quantified, thereby creating a technical barrier to CNT utilization in concrete construction. In this study, fresh OPC paste was treated as a two-phase material containing solid particles (cement grains) and liquid solutions (pore solutions). A centrifugation-based technique was proposed to separate these two phases and the presence of CNTs in each phase was quantified. UV-Vis spectrometry showed that the degree of dispersion can achieve above 90 wt% using polycarboxylate superplasticizer. The results suggested an upper limit of 0.26 wt% for CNT addition into water before mixing with OPC, and the dispersion was found to be stable for at least 4 hours. Based on scanning electron imaging, the adsorption phenomenon of CNTs on OPC grains with size less than 4 μm was discovered. Energy-dispersive X-ray spectroscopy indicated these adsorptive particles have lower Ca to Si ratio. It was observed that about 0.5 mg of CNTs per gram of OPC grains was adsorbed in solid OPC grains in typical fresh OPC pastes. On the basis of these results, a conceptual model was proposed for the distribution of CNTs in fresh OPC paste where about 33 wt% of the CNTs stay in pore solution and 65 wt% of CNTs are adsorbed on OPC grains.

 Please wait while we load your content...
Please wait while we load your content...