Hyperbranched supramolecular polymer constructed from twisted cucurbit[14]uril and porphyrin via host–guest interactions†
Zhongzheng
Gao
a,
Jing
Zhang
a,
Nana
Sun
b,
Ying
Huang
a,
Zhu
Tao
a,
Xin
Xiao
*a and
Jianzhuang
Jiang
*b
aKey Laboratory of Macrocyclic and Supramolecular Chemistry of Guizhou Province, Guizhou University, Guiyang, 550025, China. E-mail: gyhxxiaoxin@163.com
bBeijing Key Laboratory for Science and Application of Functional Molecular and Crystalline Materials, University of Science and Technology Beijing, Beijing, 100083, China. E-mail: jianzhuang@ustb.edu.cn
First published on 21st July 2016
Abstract
A novel supramolecular polymer Q[14]-TBPyP was successfully constructed from a host twisted cucurbit[14] (Q[14]) molecule and a four-arm 5,10,15,20-tetrakis(N-butyl-4-pyridinium)porphyrin tetrabromide (TBPyP) guest molecule depending on the host–guest interactions and characterized by NMR, ITC, DLS, electronic absorption, and fluorescence spectroscopy, representing the first cucurbit[14]uril-involved host–guest supramolecular polymer.
Supramolecular polymers are a special class of supramolecular architectures in which monomeric units are assembled via directional and reversible non-covalent interactions including hydrogen bonding interactions,1 strong π–π interactions,2 and metal coordination.3 The polymeric structure and dynamic non-covalent interactions render the supramolecular polymer-like nature in addition to their recycling, self-healing, adaption, and degradability properties.4 These fascinating features have attracted increasing research attention in the past several decades due to their potential applications in optoelectronic materials, drug delivery, molecular recognition systems, catalysts, and memory.5 Quite recently, a number of novel supramolecular polymers have been constructed from macrocyclic compounds such as crown ethers, calixarenes, cyclodextrins, and pillararenes depending on another kind of fascinating non-covalent driving force, the host–guest interactions, due to the unique mechanical and stimuli-responsive properties of these macrocycle-based supramolecular polymers.6
Cucurbit[n]urils (Q[n]),7 as a relatively new class of macrocyclic hosts with a tunable cavity, have been found to exhibit good host–guest supramolecular polymer formation properties because of their high binding constants with a number of guest molecules.8 Among the Q[n] homologues, both Q[6] and Q[7] are able to encapsulate the side chains of the polymeric backbone, forming “side chain” supramolecular polymers as exemplified by side chain polypseudorotaxanes.9 Interestingly, along with the increase in the cavity size to Q[8], both the “side chain” and the “main chain” supramolecular polymers were formed with small-molecular-weight monomers.10 We were inspired by the recent report on the largest cucurbit[n]uril member, cucurbit[14]uril (Q[14]),11 containing 14 normal glycoluril units linked by 28 methylene bridges with a 180° twist, and therefore possessing two cavities. Consequently, this has two cavities and adopts a folded, figure of eight conformation. In particular, it can accommodate one or two guest molecules respectively due to the two cavities.12 In the present paper, we describe the fabrication and characterization of the first Q[14]-involving the host–guest supramolecular polymer with 5,10,15,20-tetrakis(N-butyl-4-pyridinium)porphyrin tetrabromide (TBPyP) as the guest, Fig. 1.
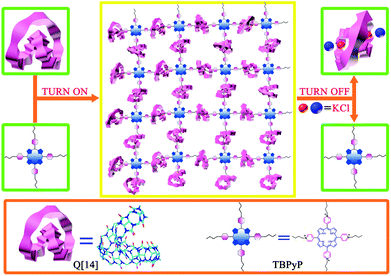 | ||
| Fig. 1 Schematic representation of the construction of the supramolecular polymer based on Q[14] and TBPyP. | ||
For the purpose of fabricating water-soluble supramolecular polymers, the 5,10,15,20-tetrakis(N-butyl-4-pyridinium)porphyrin tetrabromide (TBPyP) monomer containing four butyl-substituted arms was designed and synthesized, Fig. S1 and S2 (ESI†). The four meso-attached N-butyl-4-pyridinium moieties of TBPyP are able to bind with Q[14] to form hyperbranched supramolecular polymers, while the rigid and bulky porphyrin core can suppress the formation of cyclic species. Nevertheless, the N-butyl-4-pyridinium cation moieties incorporated into the guest compound give this compound good water-solubility, meaning that there is the opportunity for the reaction to occur with water soluble Q[14] in aqueous solution. As a consequence, simply mixing Q[14] and TBPyP in aqueous solution results in the formation of the supramolecular network Q[14]-TBPyP depending on the corresponding host–guest interaction between the two species of molecules, which was further characterized by diffusion-ordered NMR spectroscopy and dynamic light scattering analysis.
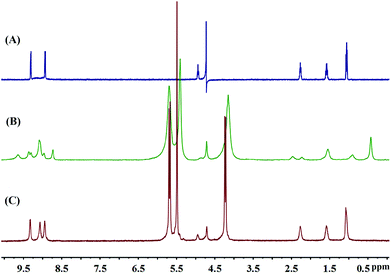
The formation of the Q[14]-TBPyP inclusion complex can be conveniently monitored by 1H NMR spectroscopy in D2O. As clearly shown in Fig. 2, along with increasing the amount of Q[14] added into the TBPyP solution in D2O, the methylene protons Ha–Hc and methyl proton Hd of the TBPyP alkyl chain experience a significant upfield shift. However, this is definitely not true for the pyridinium protons of the guest TBPyP, the signal at 9.32 ppm attributed to the pyridinium porton Hα in the free guest TBPyP experiences a considerable up-field shift after the addition of Q[14], and meanwhile another pyridinium porton Hβ with the signal at 8.94 ppm experiences a down-field shift. Notably, along with increasing the amount of Q[14] added into the TBPyP solution, two sets of signals of the guest TBPyP appeared, indicating that the alkyl chain and the partial pyridyl moiety of the guest TBPyP were bound within two different cavity environments of the host Q[14] in the complex Q[14]-TBPyP supramolecular structure. This is further confirmed by the NOESY cross-signals between the alkyl chain and pyridyl pyridinium protons of the guest TBPyP and methylene proton-bridged glycoluril dimmers of Q[14] in the NOESY spectrum of the mixture of Q[14] and TBPyP in a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in D2O, Fig. S3 (ESI†).
1 in D2O, Fig. S3 (ESI†).
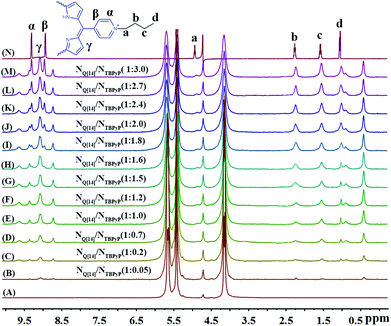 | ||
| Fig. 2 Titration 1H NMR spectra for Q[14] (A) in the presence of various equivalents of TBPyP (B–M) and neat TBPyP (N) in D2O. | ||
Isothermal titration calorimetry (ITC) measurements are able to afford quantitative information on the host–guest complexation including both the binding affinity and thermodynamic origin. As a consequence, a solution of Q[14] in H2O was consecutively injected into a solution of TBPyP in H2O to record the exothermic binding isotherm, Fig. S6 (ESI†), resulting in the resolution of the binding molar ratio value of N = 2.087. This suggests that the binding stoichiometry of Q[14] to TBPyP is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In addition, the association constant of Ka = (1.97 ± 0.14) × 105 M−2 for Q[14] with TBPyP was also derived on the basis of the corresponding experimental result. Such a high binding constant indicates the relatively strong host–guest interaction between Q[14] and TBPyP, rationalizing the construction of a stable supramolecular hyperbranched polymer of Q[14]-TBPyP.
1. In addition, the association constant of Ka = (1.97 ± 0.14) × 105 M−2 for Q[14] with TBPyP was also derived on the basis of the corresponding experimental result. Such a high binding constant indicates the relatively strong host–guest interaction between Q[14] and TBPyP, rationalizing the construction of a stable supramolecular hyperbranched polymer of Q[14]-TBPyP.
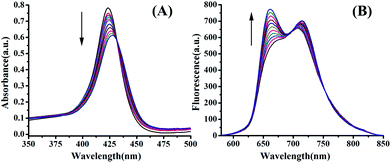
Both electronic absorption and fluorescence emission spectroscopies were utilized to collect further information about the binding between Q[14] and TBPyP. According to the electronic absorption spectroscopy results, Fig. 3(A), upon the gradual addition of Q[14] in H2O into TBPyP in H2O, the porphyrin Soret absorption underwent a slight bathochromic shift from 422 to 429 nm in addition to a significant decrease in its intensity due to the formation of the complex Q[14]-TBPyP. Further support for this conclusion comes from the fluorescence spectroscopy result and the observation of an increase in the emission intensity, accompanied by a slight hypochromatic shift in the porphyrin Q band, after adding Q[14] into the solution of TBPyP, Fig. 3(B).
Diffusion-ordered NMR spectroscopy (DOSY) can correlate the 1H NMR signals with the diffusion coefficient (D) in solution and therefore has been successfully used to characterize the formation of supramolecular polymers.13 As a consequence, in the present case the average diffusion coefficients (Davg) of the host Q[14] and the free guest TBPyP were determined to be 2.82 × 10−10 and 2.97 × 10−10 m2 s−1, respectively, according to DOSY. However, in the mixture of Q[14] and TBPyP in D2O with a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the NMR signals of both Q[14] and TBPyP show only a single diffusion coefficient with a significantly decreased value of Davg = 9.11 × 10−11 m2 s−1, implying the formation of a large-size hyperbranched supramolecular polymer. Nevertheless, due to the involvement of the two building blocks of Q[14] and TBPyP, the supramolecular polymerization is certainly able to be controlled via tuning the ratio between Q[14] and TBPyP. DOSY spectroscopy results of a series of samples with different ratios between Q[14] and TBPyP in D2O were then recorded. As displayed in Fig. S7 (ESI†), along with the increase in the ratio of Q[14] to TBPyP, the diffusion coefficient of the complex decreases with the lowest diffusion coefficient being reached at the molar ratio between Q[14] and TBPyP of 2.0, indicative of the supramolecular hyperbranched polymer with the highest molecular weight at this point.
1, the NMR signals of both Q[14] and TBPyP show only a single diffusion coefficient with a significantly decreased value of Davg = 9.11 × 10−11 m2 s−1, implying the formation of a large-size hyperbranched supramolecular polymer. Nevertheless, due to the involvement of the two building blocks of Q[14] and TBPyP, the supramolecular polymerization is certainly able to be controlled via tuning the ratio between Q[14] and TBPyP. DOSY spectroscopy results of a series of samples with different ratios between Q[14] and TBPyP in D2O were then recorded. As displayed in Fig. S7 (ESI†), along with the increase in the ratio of Q[14] to TBPyP, the diffusion coefficient of the complex decreases with the lowest diffusion coefficient being reached at the molar ratio between Q[14] and TBPyP of 2.0, indicative of the supramolecular hyperbranched polymer with the highest molecular weight at this point.
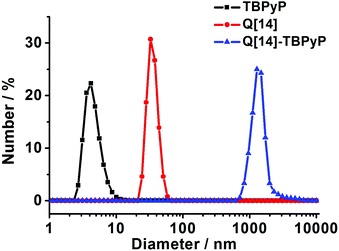
Dynamic light scattering (DLS) performed in dilute solution has quite often been employed to monitor the formation of a supramolecular polymer. Fig. 4 shows the DLS data of the supramolecular polymer Q[14]-TBPyP in water. As can be found, mixing Q[14] and TBPyP at a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (5.0 mM) leads to the observation of one hydrodynamic diameter distribution centered at 1281 nm, indicating the formation of the aggregates with average diameters of this size in water solution. This gives unambiguous evidence for the formation of supramolecular polymer architectures between Q[14] and TBPyP.
1 (5.0 mM) leads to the observation of one hydrodynamic diameter distribution centered at 1281 nm, indicating the formation of the aggregates with average diameters of this size in water solution. This gives unambiguous evidence for the formation of supramolecular polymer architectures between Q[14] and TBPyP.
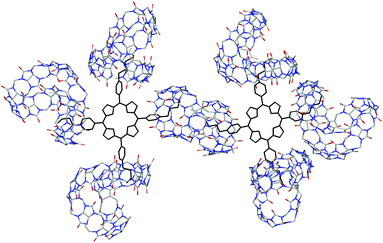
At the end of this section, it is worth noting that despite numerous attempts, all the efforts made thus far failed to grow X-ray quality single crystals of the supramolecular complex. As a result, molecular modeling using density functional theory (DFT) calculations at the B3LYP/6-31G (D, P) level was employed to provide the structural information for the supramolecular polymer, Fig. 5.
It is well known that the reversible transformation to and from the starting building block in particular for the guest species under certain conditions is the primary feature of supramolecular polymers.14 This is also true for the present case with the addition of KCl as the competitive compound due to the strong coordination ability of potassium ions with the carbonyl groups of Q[14]. As shown in Fig. 6, upon adding KCl into the Q[14]-TBPyP supramolecular polymer solution in D2O, the signals of TBPyP in the Q[14]-TBPyP system returned to the original state of the free TBPyP molecule, indicating the effective dissociation of the original Q[14]-TBPyP supramolecular polymer with the re-formation of the guest TBPyP species and concomitant formation of the new inclusion complex between Q[14] and KCl. Further support for this point comes from the electronic absorption and fluorescence spectroscopy results. Upon addition of KCl into the above-mentioned Q[14]-TBPyP supramolecular polymer system, both the electronic absorption and fluorescence spectra recovered to the original state of the free TBPyP molecule, confirming the dissociation of the Q[14]-TBPyP supramolecular polymer, Fig. S8–S10 (ESI†).
In summary, a novel supramolecular polymer was successfully constructed between cucurbit[14]uril and 5,10,15,20-tetrakis(N-butyl-4-pyridinium)porphyrin tetrabromide depending on the host–guest interactions, which was characterized by the combination of various techniques including NMR, electronic absorption, fluorescence spectroscopy and DLS, representing the first example of a cucurbit[14]uril-involved supramolecular polymer. Nevertheless, addition of the competitive guest compound KCl led to effective dissociation of the supramolecular polymer Q[14]-TBPyP and re-formation of the original guest species, giving additional support for the host–guest interaction-based supramolecular polymer nature of the present system.
Acknowledgements
Financial support from the National Key Basic Research Program of China (Grant No. 2013CB933402), the Natural Science Foundation of China (21561007 and 21562015), the Beijing Municipal Commission of Education, and the University of Science and Technology Beijing is gratefully acknowledged.Notes and references
- (a) K. C.-F. Leung, P. M. Mendes, S. N. Magonov, B. H. Northrop, S. Kim, K. Patel, A. H. Flood, H.-R. Tseng and J. F. Stoddart, J. Am. Chem. Soc., 2006, 128, 10707 CrossRef CAS PubMed; (b) E. M. Todd and S. C. Zimmerman, Tetrahedron, 2008, 64, 8558 CrossRef CAS; (c) P. Shokrollahi, H. Mirzadeh, W. T. S. Huck and O. A. Scherman, Polymer, 2010, 51, 6303 CrossRef CAS; (d) J. L. Wietor, D. J. M. van Beek, G. W. Peters, E. Mendes and R. P. Sijbesma, Macromolecules, 2011, 44, 1211 CrossRef CAS; (e) X. Z. Yan, S. J. Li, J. B. Pollock, T. R. Cook, J. Z. Chen, Y. Y. Zhang, X. F. Ji, Y. H. Yu, F. H. Huang and P. J. Stang, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 15585 CrossRef CAS PubMed; (f) T. Rossow, S. Hackelbusch, P. V. Assenbergh and S. Seiffert, Polym. Chem., 2013, 4, 2515 RSC.
- C. C. Lee, C. Grenier, E. W. Meijer and A. P. H. J. Schenning, Chem. Soc. Rev., 2009, 38, 671 RSC.
- (a) W. Weng, J. B. Beck, A. M. Jamieson and S. J. Rowan, J. Am. Chem. Soc., 2006, 128, 11663 CrossRef CAS PubMed; (b) G. Schwarz, Y. Bodenthin, Z. Tomkowicz, W. Haase, T. Geue, J. Kohlbrecher, U. Pietsch and D. G. Kurth, J. Am. Chem. Soc., 2011, 133, 547 CrossRef CAS PubMed; (c) D. Xu and S. L. Craig, Macromolecules, 2011, 44, 5465 CrossRef CAS PubMed; (d) Y. K. Tian, L. Chen, Y. J. Tian, X. Y. Wang and F. Wang, Polym. Chem., 2013, 4, 453 RSC; (e) X. Z. Yan, J. F. Xu, T. R. Cook, F. H. Huang, Q. Z. Yang, C. H. Tung and P. J. Stang, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 8717 CrossRef CAS PubMed.
- (a) R. Hoogenboom and U. S. Schubert, Chem. Soc. Rev., 2006, 35, 622 RSC; (b) A. Harada, Y. Takashima and H. Yamaguchi, Chem. Soc. Rev., 2009, 38, 875 RSC; (c) B. Zheng, F. Wang, S. Dong and F. Huang, Chem. Soc. Rev., 2012, 41, 1621 RSC; (d) Y. Liu, Z. Wang and X. Zhang, Chem. Soc. Rev., 2012, 41, 5922 RSC; (e) S. L. Li, T. Xiao, C. Lin and L. Wang, Chem. Soc. Rev., 2012, 41, 5950 RSC; (f) E. A. Appel, J. Del Barrio, X. J. Loh and O. A. Scherman, Chem. Soc. Rev., 2012, 41, 6195 RSC; (g) X. Yan, F. Wang, B. Zheng and F. Huang, Chem. Soc. Rev., 2012, 41, 6042 RSC; (h) R. Fang, Y. Liu, Z. Wang and X. Zhang, Polym. Chem., 2013, 4, 900 RSC; (i) P. Wei, X. Yan and F. Huang, Chem. Soc. Rev., 2015, 44, 815 RSC; (j) C. Heinzmann, C. Weder and L. M. Espinosa, Chem. Soc. Rev., 2016, 45, 342 RSC; (k) E. Krieg, M. M. C. Bastings, P. Besenius and B. Rybtchinski, Chem. Rev., 2016, 116, 2414 CrossRef CAS PubMed.
- (a) A. Ajayaghosh, R. Varghese, V. Praveen and S. Mahesh, Angew. Chem., Int. Ed., 2006, 45, 3261 CrossRef CAS PubMed; (b) V. Praveen, S. George, R. Varghese, C. Vijayakumar and A. Ajayaghosh, J. Am. Chem. Soc., 2006, 128, 7542 CrossRef CAS PubMed; (c) Z. Zhang, Y. Luo, J. Chen, S. Dong, Y. Yu, Z. Ma and F. Huang, Angew. Chem., Int. Ed., 2011, 50, 1397 CrossRef CAS PubMed; (d) S. Babu, S. Prasanthkumar and A. Ajayaghosh, Angew. Chem., Int. Ed., 2012, 51, 1766 CrossRef CAS PubMed; (e) Y. Li, T. Liu, H. Liu, M. Tian and Y. Li, Acc. Chem. Res., 2014, 47, 1186 CrossRef CAS PubMed; (f) B. Shi, K. Jie, Y. Zhou, D. Xia and Y. Yao, Chem. Commun., 2015, 51, 4503 RSC.
- (a) F. Wang, J. Zhang, X. Ding, S. Dong, M. Liu, B. Zheng, S. Li, L. Wu, Y. Yu, H. W. Gibson and F. Huang, Angew. Chem., Int. Ed., 2010, 49, 1090 CrossRef CAS PubMed; (b) Z. Niu, F. Huang and H. W. Gibson, J. Am. Chem. Soc., 2011, 133, 2836 CrossRef CAS PubMed; (c) X. Yan, M. Zhou, J. Chen, X. Chi, S. Dong, M. Zhang, X. Ding, Y. Yu, S. Shao and F. Huang, Chem. Commun., 2011, 47, 7086 RSC; (d) S. Y. Dong, Y. Luo, X. Z. Yan, B. Zheng, X. Ding, Y. H. Yu, Z. Ma, Q. L. Zhao and F. H. Huang, Angew. Chem., Int. Ed., 2011, 50, 1905 CrossRef CAS PubMed; (e) B. Xia, B. Zheng, C. Han, S. Dong, M. Zhang, B. Hu, Y. Yu and F. Huang, Polym. Chem., 2013, 4, 2019 RSC; (f) X. Xiao, J. S. Sun and J. Z. Jiang, Chem. – Eur. J., 2013, 19, 16891 CrossRef CAS PubMed; (g) X. Xiao, R. Lin, L. Zheng, W. Sun, Z. Tao, S. Xue, Q. Zhu and J. Liu, RSC Adv., 2014, 4, 53665 RSC; (h) X. Xiao, N. Sun, D. Qi and J. Jiang, Polym. Chem., 2014, 5, 5211 RSC; (i) X. Ji, K. Jie, S. C. Zimmerman and F. Huang, Polym. Chem., 2015, 6, 1912 RSC; (j) C. Sun, J. Xu, Y. Chen, L. Niu, L. Wu, C. H. Tung and Q. Yang, Polym. Chem., 2016, 7, 2057 RSC; (k) Z. Huang, Y. Fang, Q. Luo, S. Liu, G. An, C. Hou, C. Lang, J. Xu, Z. Dong and J. Liu, Chem. Commun., 2016, 52, 2083 RSC; (l) Z. Zhou, X. Yan, T. R. Cook, M. L. Saha and P. J. Stang, J. Am. Chem. Soc., 2016, 138, 806 CrossRef CAS PubMed.
- (a) A. I. Day, A. P. Arnold and R. J. Blanch, WO2000/068232, 2000 Search PubMed; (b) J. Lagona, P. Mukhopadhyay, S. Chakrabarti and L. Isaacs, Angew. Chem., Int. Ed., 2005, 44, 4844 CrossRef CAS PubMed; (c) E. Masson, X. Ling, R. Joseph, L. Kyeremeh-Mensah and X. Lu, RSC Adv., 2012, 2, 1213 RSC; (d) X. L. Ni, X. Xiao, H. Cong, Q. J. Zhu, S. F. Xue and Z. Tao, Acc. Chem. Res., 2014, 47, 1386 CrossRef CAS PubMed.
- (a) K. Kim, N. Selvapalam, Y. H. Ko, K. M. Park, D. Kim and J. Kim, Chem. Soc. Rev., 2007, 36, 267 RSC; (b) X. Xiao, J. X. Liu, Z. F. Fan, K. Chen, Q. J. Zhu, S. F. Xue and Z. Tao, Chem. Commun., 2010, 46, 3741 RSC; (c) X. Xiao, W. J. Li and J. Z. Jiang, Inorg. Chem. Commun., 2013, 35, 156 CrossRef CAS; (d) X. Xiao, S. C. Tu, S. F. Xue, Q. J. Zhu, Z. Tao, J. X. Zhang and G. Wei, Chem. J. Chin. Univ., 2013, 34, 354 CAS; (e) B. Yang, X. Xiao, Y. Q. Zhang, Q. J. Zhu, S. F. Xue, Z. Tao and G. Wei, RSC Adv., 2014, 4, 44359 RSC; (f) B. Yang, L. M. Zheng, Z. Z. Gao, X. Xiao, Q. J. Zhu, S. F. Xue, Z. Tao, J. X. Liu and G. Wei, J. Org. Chem., 2014, 79, 11194 CrossRef CAS PubMed; (g) X. Xiao, Z. Z. Gao, C. L. Shan, Z. Tao, Q. J. Zhu, S. F. Xue and J. X. Liu, Phys. Chem. Chem. Phys., 2015, 17, 8618 RSC; (h) K. I. Assaf and W. M. Nau, Chem. Soc. Rev., 2015, 44, 39 RSC.
- (a) A. Corma, H. Garcia and P. Montes-Navajas, Tetrahedron Lett., 2007, 48, 4613 CrossRef CAS; (b) Y. Liu, C. F. Ke, H. Y. Zhang, W. J. Wu and J. Shi, J. Org. Chem., 2007, 72, 280 CrossRef CAS PubMed; (c) S. W. Choi and H. Ritter, Macromol. Rapid Commun., 2007, 28, 101 CrossRef CAS; (d) J. J. Reczek, A. A. Kennedy, B. T. Halbert and A. R. Urbach, J. Am. Chem. Soc., 2009, 131, 2408 CrossRef CAS PubMed; (e) E. A. Appel, F. Biedermann, U. Rauwald, S. T. Jones, J. M. Zayed and O. A. Scherman, J. Am. Chem. Soc., 2010, 132, 14251 CrossRef CAS PubMed; (f) H. Yang, H. J. Cheng and Y. B. Tan, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 2138 CrossRef CAS.
- (a) Y. L. Liu, Y. Yu, J. Gao, Z. Q. Wang and X. Zhang, Angew. Chem., 2010, 122, 6726 ( Angew. Chem., Int. Ed. , 2010 , 49 , 6576 ) CrossRef; (b) D. Z. Jiao, F. Biedermann, F. Tian and O. A. Scherman, J. Am. Chem. Soc., 2010, 132, 15734 CrossRef CAS PubMed; (c) Y. L. Liu, Z. Q. Wang and X. Zhang, Chem. Soc. Rev., 2012, 41, 5922 RSC; (d) Y. L. Liu, R. C. Fang, X. X. Tan, Z. Q. Wang and X. Zhang, Chem. – Eur. J., 2012, 18, 15650 CrossRef CAS PubMed; (e) R. C. Fang, Y. L. Liu, Z. Q. Wang and X. Zhang, Polym. Chem., 2013, 4, 900 RSC.
- X. J. Cheng, L. L. Liang, K. Chen, N. N. Ji, X. Xiao, J. X. Zhang, Y. Q. Zhang, S. F. Xue, Q. J. Zhu, X. L. Ni and Z. Tao, Angew. Chem., Int. Ed., 2013, 52, 7252 CrossRef CAS PubMed.
- (a) Q. Liu, Q. Li, X. J. Cheng, Y.-Y. Xi, B. Xiao, X. Xiao, Q. Tang, Y. Huang, Z. Tao, S. F. Xue, Q. J. Zhu and J.-X. Zhang, Chem. Commun., 2015, 51, 9999 RSC; (b) Q. Li, S. C. Qiu, K. Chen, Y. Zhang, R. B. Wang, Y. Huang, Z. Tao, Q. J. Zhu and J. X. Liu, Chem. Commun., 2016, 52, 2589 RSC.
- (a) Q. Wang, Y. Chen and Y. Liu, Polym. Chem., 2013, 4, 4192 RSC; (b) Y. L. Liu, Z. H. Huang, X. X. Tan, Z. Q. Wang and X. Zhang, Chem. Commun., 2013, 49, 5766 RSC; (c) L. L. Yang, X. X. Tan, Z. Q. Wang and X. Zhang, Chem. Rev., 2015, 115, 7196 CrossRef CAS PubMed; (d) X. G. Liu, J. F. Xu, Z. Q. Wang and X. Zhang, Polym. Chem., 2016, 7, 2333 RSC.
- (a) B. J. B. Folmer, R. P. Sijbesma and E. W. Meijer, J. Am. Chem. Soc., 2001, 123, 2093 CrossRef CAS PubMed; (b) S.-L. Li, T.-X. Xiao, B.-J. Hu, Y.-J. Zhang, F. Zhao, Y. Ji, Y.-H. Yu, C. Lin and L.-Y. Wang, Chem. Commun., 2011, 47, 10755 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis procedure, 1H NMR and 1H–1H COSY NMR, NOSEY, COSY, DOSY and ITC results. See DOI: 10.1039/c6qo00310a |
| This journal is © the Partner Organisations 2016 |